Human DNA Vaccines Market Size and Forecast – 2025 – 2032
The Global Human DNA Vaccines Market size is estimated to be valued at USD 1.85 billion in 2025 and is expected to reach USD 5.60 billion by 2032, exhibiting a compound annual growth rate (CAGR) of 16.4% from 2025 to 2032.
Global Human Dna Vaccines Market Overview
Human DNA vaccines are a next-generation immunization approach that uses engineered DNA to stimulate the body's immune response. Instead of introducing a weakened or inactivated pathogen, DNA vaccines deliver genetic instructions (usually via a plasmid) that enable human cells to produce specific proteins resembling components of a virus or bacteria. These proteins function as antigens that are recognized by the immune system, triggering both antibody-mediated and cellular immune responses. This method allows for targeted and precise stimulation of immunity, with the added benefits of faster development timelines, simplified production, and enhanced stability compared to traditional vaccines.
DNA vaccines also offer flexibility in design and can accommodate multiple antigen targets in a single dose. They are currently being researched for various infectious diseases such as HIV, Zika, and influenza, and show potential in areas like cancer therapy and allergy prevention. Despite these advantages, challenges remain, particularly in achieving efficient delivery into human cells and maintaining a strong, long-lasting immune response. New delivery techniques like electroporation and nanoparticle-based carriers are being explored to address these limitations. As research progresses, DNA vaccines are increasingly recognized for their adaptability and potential to revolutionize global vaccination strategies.
Key Takeaways
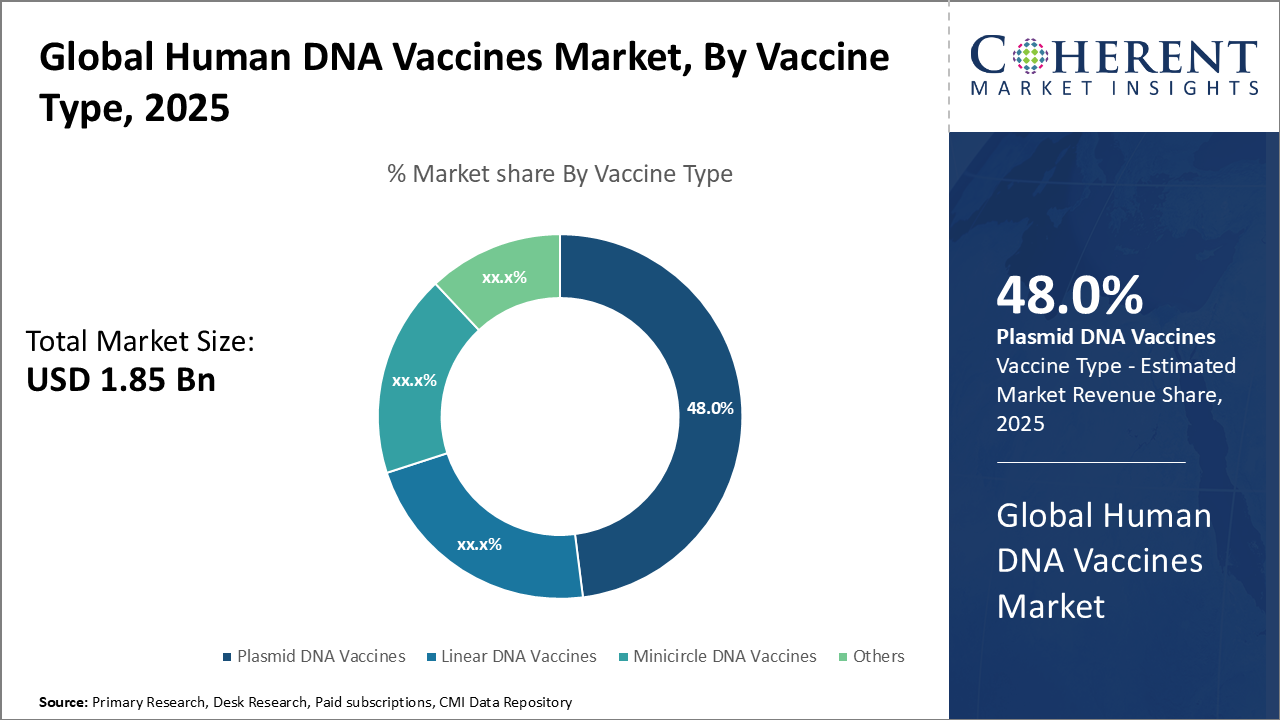
In the vaccine type segment, Plasmid DNA vaccines dominate with a 48% share due to efficacy and scalability. Linear DNA vaccines grow fastest, supporting personalized medicine, while Minicircle vaccines target oncology. Others include early-stage emerging DNA constructs.
In the application segment, Infectious diseases lead the market with 55% revenue, driven by HPV, Zika, and influenza vaccinations. Oncology grows fastest with DNA-based cancer immunotherapies. Autoimmune applications and emerging uses in veterinary medicine and rare genetic disorders diversify the market.
In the end user segment, Hospitals lead the market with over 50% utilization due to their central role in vaccine administration. Clinics are the fastest-growing segment, while research institutes drive innovation. Others include government programs and alternative providers enhancing DNA vaccine access.
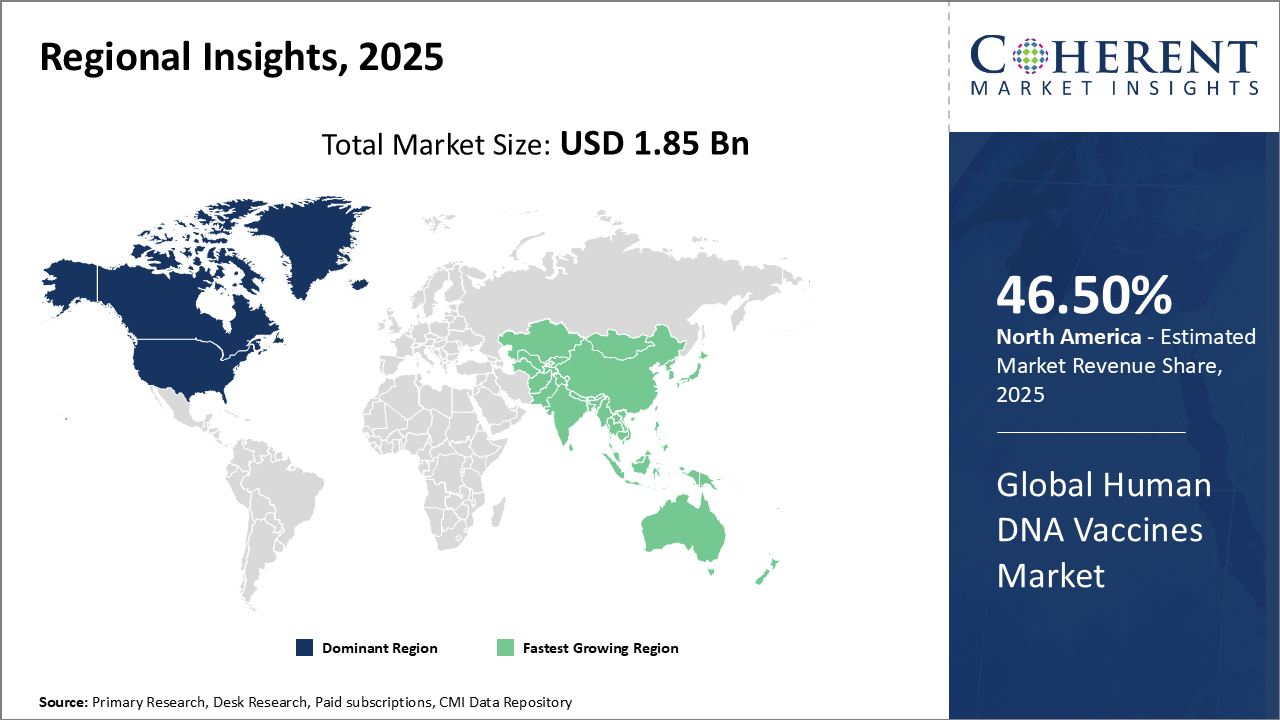
North America dominates the Human DNA Vaccines market with strong R&D, public health programs, and key players like Moderna. Asia Pacific grows fastest, driven by expanding infrastructure, cost-effective manufacturing, government incentives, and local partnerships.
The USA leads the Human DNA Vaccines market with strong biotech investment and innovation, while India grows rapidly due to affordable vaccine demand, local manufacturing, government support, and expanding clinical pipelines.
Human DNA Vaccines Market Segmentation Analysis
To learn more about this report, Download Free Sample
Human DNA Vaccines Market Insights, By Vaccine Type
Plasmid DNA vaccines dominate the market with a 48% share due to their proven efficacy, stability, and scalable production, making them the preferred choice for infectious disease prevention. Linear DNA vaccines are the fastest-growing subsegment, driven by simpler manufacturing and improved safety, supporting their rise in personalized medicine. Minicircle DNA vaccines serve niche therapeutic areas with high transgene expression and low immunogenicity, especially in oncology. The Others segment includes emerging DNA vaccine constructs still in early development.
Human DNA Vaccines Market Insights, By Application
Infectious diseases dominate the market with nearly 55% of revenue, driven by extensive vaccination efforts targeting HPV, Zika, and influenza. Oncology is the fastest-growing segment due to rising demand for DNA-based cancer immunotherapies that trigger targeted tumor responses. Autoimmune disorder applications are also gaining traction as DNA vaccines are developed to regulate immune activity. The Others segment includes emerging uses in veterinary medicine and rare genetic disorders, contributing to a diversified market landscape.
Human DNA Vaccines Market Insights, By End-User
Hospitals dominate the market with over 50% utilization, reflecting their primary role in vaccine administration and post-market monitoring. Clinics are the fastest-growing subsegment, driven by a shift toward outpatient services and personalized vaccination schedules. Research institutes significantly contribute to market expansion by advancing clinical trials and developing new vaccine pipelines. The Others segment includes government immunization programs and alternative healthcare providers that enhance access to DNA vaccines across diverse settings.
Human DNA Vaccines Market Trends
The market is shifting toward personalized immunotherapies as DNA vaccines are increasingly customized to target individual immune profiles, especially in oncology and autoimmune treatments.
Combination DNA vaccines integrated with immune-boosting adjuvants are gaining momentum, with 2024 clinical trials reporting up to 35% higher efficacy than traditional standalone DNA vaccines.
Advanced genome editing technologies like CRISPR are being applied to enhance plasmid DNA stability and expression efficiency, driving innovation in vaccine design and effectiveness.
Decentralized and modular manufacturing approaches are emerging to enable localized production, enhancing scalability and rapid deployment during outbreaks or pandemics.
Growth in cold-chain independent formulations is reducing logistical barriers, making DNA vaccines more accessible in regions with limited refrigeration infrastructure and supporting broader global vaccination coverage.
Human DNA Vaccines Market Insights, By Geography
To learn more about this report, Download Free Sample
North America Human DNA Vaccines Market Analysis and Trends
North America dominates the Human DNA Vaccines market due to its mature pharmaceutical ecosystem, annual R&D spending exceeding USD 2 billion, and strong integration of DNA vaccines into public health programs. The U.S. leads this growth with extensive clinical pipelines and government initiatives targeting HPV variants. Advanced research infrastructure further accelerates vaccine development and commercialization, supported by key players such as Moderna Therapeutics and VGXI Inc., reinforcing the region’s market leadership.
Asia Pacific Human DNA Vaccines Market Analysis and Trends
Asia Pacific is the fastest-growing region in the Human DNA Vaccines market, with a CAGR exceeding 18%, driven by expanding healthcare infrastructure and cost-efficient manufacturing capabilities. China and India lead this growth, fueled by large-scale immunization efforts and government incentives promoting vaccine innovation. Supportive regulatory frameworks encourage R&D and clinical advancements, while companies increasingly leverage local partnerships and technology transfers to strengthen regional presence and capitalize on rising market demand.
Human DNA Vaccines Market Outlook for Key Countries
USA Human DNA Vaccines Market Analysis and Trends
The USA leads the Human DNA Vaccines market due to robust biotechnology investments and strong precision medicine initiatives. In 2024, NIH funding rose by 20%, accelerating vaccine pipelines targeting cancer and infectious diseases. Key players like Moderna Therapeutics and Inovio Pharmaceuticals drive innovation through rapid clinical trials and advanced delivery technologies. The country’s sophisticated healthcare system and focus on high-risk populations further support widespread adoption of DNA vaccines, solidifying the U.S. as a global leader in this sector.
India Human DNA Vaccines Market Analysis and Trends
India’s Human DNA Vaccines market is rapidly growing, fueled by rising demand for affordable vaccines and expanding local manufacturing capabilities. Government initiatives like “Make in India” have attracted global investments, strengthening domestic production. Companies such as Zydus Cadila and GeneOne Life Science are advancing clinical trial pipelines, supported by increased healthcare spending and broader immunization campaigns. These factors position India as a key growth hub in the Asia Pacific region, contributing significantly to market expansion.
Analyst Opinion
The Human DNA Vaccines market is being significantly influenced by increased production capacity, a crucial supply-side factor. In 2024, global production grew by over 30% due to advancements in vector design and synthesis, especially in North America, which helped accelerate clinical progress and regulatory success.
Demand has surged from emerging infectious disease segments, with Asia Pacific reporting a 25% rise in import volumes during 2024–2025. Applications in oncology and personalized medicine have broadened revenue streams, with high demand for HPV and Zika DNA vaccines in Latin America.
Pricing strategies have evolved in developed markets like the U.S. and Germany, where increased flexibility followed successful Phase III trials. A 12% drop in average vaccine costs in 2024 contributed to wider public and private adoption.
Technological advancements, including decentralized trials and nanoparticle-based delivery systems, are reshaping market dynamics. By early 2025, nanoparticle use in formulations rose by 40%, boosting immunogenicity and supporting competitive positioning.
Market Scope
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2025 | Market Size in 2025: |
USD 1.85 billion |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 16.4% | 2032 Value Projection: | USD 5.6 billion |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: | Moderna Therapeutics, Inovio Pharmaceuticals, GeneOne Life Science, Zydus Cadila Healthcare, Dynavax Technologies, Genexine Inc., Shenzhen Geno-Immune Medical Institute, Applied DNA Sciences, Immunomic Therapeutics, Vaxart Inc., VGXI Inc., AnGes Inc. | ||
| Growth Drivers: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Human DNA Vaccines Market Growth Factors
The increasing susceptibility of the global population to viral outbreaks is a key driver for the Human DNA Vaccines market, prompting governments to prioritize research and development. In 2024 alone, over USD 500 million in grants were allocated worldwide to support DNA vaccine initiatives. Technological advancements in gene synthesis and innovative delivery systems have lowered production costs, making DNA vaccines more accessible and expanding market potential. Growing use in oncology is another significant growth factor, as these vaccines can trigger strong immune responses against tumor-specific antigens. Additionally, the expansion of healthcare infrastructure in regions like Asia Pacific and Latin America is creating new opportunities, enabling broader vaccine distribution and driving market revenue growth.
Human DNA Vaccines Market Development
In February 2025, Maravai LifeSciences, Inc. has completed its acquisition of Officinae Bio’s DNA and RNA business. This deal merges Officinae Bio’s AI-powered mRNA design platform with Maravai and TriLink BioTechnologies’ top drug manufacturing capabilities, giving customers advanced expertise and innovative technologies to accelerate mRNA sequence optimization, moving efficiently from development to clinical trials and commercial production.
In June 2025, PharmaJet and Immuno Cure partnered to advance ICVAX, a therapeutic HIV DNA vaccine, using PharmaJet’s needle-free Tropis intradermal system. This delivery enhances immune responses and patient experience. Following promising Phase 1 results, the collaboration aims to improve vaccine performance. Tropis, WHO-prequalified and commercially scaled, supports broader adoption, marking a key step in DNA vaccine innovation for infectious disease treatment.
Key Players
Leading Companies of the Market
Moderna Therapeutics
Inovio Pharmaceuticals
GeneOne Life Science
Zydus Cadila Healthcare
Dynavax Technologies
Genexine Inc.
Shenzhen Geno-Immune Medical Institute
Applied DNA Sciences
Immunomic Therapeutics
Vaxart Inc.
VGXI Inc.
AnGes Inc.
Many of these companies are pursuing strategic collaborations to accelerate clinical pipelines. For example, Inovio Pharmaceuticals partnered with a major pharmaceutical firm in mid-2024, resulting in expedited Phase II trials for its Zika DNA vaccine candidate, which substantially increased its market penetration. Furthermore, Moderna expanded manufacturing capabilities by integrating modular production lines in early 2025, effectively reducing lead times and increasing vaccine output by 22%.
Human DNA Vaccines Market Future Outlook
The Human DNA Vaccines market is set for strong growth, driven by rising demand for safe and rapid immunization solutions. Advances in delivery technologies, including electroporation and nanoparticle carriers, are enhancing efficacy and patient compliance. Personalized medicine applications, particularly in oncology and autoimmune disorders, are expanding market opportunities. Emerging regions like Asia Pacific and Latin America are witnessing rapid growth due to improved healthcare infrastructure and supportive government initiatives. Integration of genome editing tools like CRISPR is expected to further optimize vaccine performance, shaping a promising future for the market.
Human DNA Vaccines Market Historical Analysis
Over the past decade, the Human DNA Vaccines market has steadily grown, driven by advances in biotechnology and rising awareness of preventive healthcare. Early efforts focused on plasmid-based vaccines for infectious diseases, with North America and Europe leading clinical trials. Initial challenges, such as low delivery efficiency and limited immune responses, were addressed through innovations in electroporation and viral vector technologies. Between 2015 and 2023, increased R&D investment, frequent viral outbreaks, and expanding immunization programs in emerging markets fueled market growth, while key players like Moderna and Inovio advanced vaccines from research to commercialization.
Sources
Primary Research Interviews:
Biotech Researchers
Vaccine Developers
Clinical Trial Coordinators
Pharmaceutical Manufacturing Experts
Magazines:
BioPharma Dive
Vaccine News Daily
Genetic Engineering & Biotechnology News
PharmaTimes
Journals:
Human Vaccines & Immunotherapeutics
Vaccine
Frontiers in Immunology
Journal of Translational Medicine
Associations:
International Society for Vaccines (ISV)
Biotechnology Innovation Organization (BIO)
Coalition for Epidemic Preparedness Innovations (CEPI)
World Health Organization (WHO) – Vaccine Research Division
Share
Share
About Author
Vipul Patil is a dynamic management consultant with 6 years of dedicated experience in the pharmaceutical industry. Known for his analytical acumen and strategic insight, Vipul has successfully partnered with pharmaceutical companies to enhance operational efficiency, cross broader expansion, and navigate the complexities of distribution in markets with high revenue potential.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients