HPAPIs are characterized by the occupational exposure limit (OEL) of at or lower than 10 micrograms per cubic meter of air in micrograms / meter cube. The lower the value of OEL, the more potent is the compound that is being manufactured. Antibody drug conjugates (ADCs) are one of the example for HPAPIs.
The following table shows the operator exposure as per the OEL/OEB values along with the appropriate Equipment Supply Co. (ESCO) equipment for containment.
| BAND | OEB 1 | OEB 2 | OEB 3 | OEB 4 | OEB 5 | OEB 6 | OEB 7 | ||||
| OEL | >1000 - 5000 µg/m3 | >100 - ≤1000 µg/m3 | >10 - ≤100 µg/m3 | >1 - ≤10 µg/m3 | <1.0 - 0.01 µg/m3 | 0.01 - 0.001 µg/m3 |
<0.001 µg/m3 - <1 ng/m3 |
||||
| Equipment to use | Ventilation Contain-ment | Ventilation Contain-ment or Flowhoods without downflow (single pass Fume cabinets) | Downflow Booths or VBEs, Flowhoods | VBEs or DFBs with higher containment, Flowhoods with downflow and inflow for small volume | Isolators recommended however if handling less than 3kg and short task duration, low dust cloud potential reverse oRABs possible | Isolators |
Isolators |
||||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Global HPAPIs and Cytotoxic Drugs Manufacturing Market - Impact of the Coronavirus (COVID-19) Pandemic
The COVID-19 pandemic is expected to increase the growth of the global HPAPIs and cytotoxic drugs manufacturing market, owing to the increase signing of the agreements by the market players. For instance, in 2021, Ajinomoto Bio-Pharma Services, a leading provider of biopharmaceutical contract development and manufacturing services, and Humanigen, Inc. a clinical-stage biopharmaceutical company, entered into agreement and is engaged in the development of its lead drug candidate lenzilumab. Under this agreement, Aji Bio-Pharma will help in the supply chain for Humanigen and Humanigen is continuing the active enroll of the patients in a Phase III clinical trial in the U.S. and Brazil and also preparing for a potential COVID-19 Emergency Use Authorization (EUA) for lenzilumab.
Supply chain and manufacturing activities in North America, Asia Pacific, Europe, and other regions have been disrupted due to the lockdown. Furthermore, players operating in the global HPAPIs and cytotoxic drugs manufacturing market are facing major challenges on various fronts due to the COVID-19 pandemic. The major challenges include supply of raw materials required for manufacturing HPAPIs and cytotoxic drugs and due to irregularities in transportation facility. Moreover, pharmaceutical product distributors are experiencing irregular demand for products from the end users due to increasing number of patients suffering from COVID-19 and other life threatening disorders.
The global HPAPIs and cytotoxic drugs manufacturing market is estimated to be valued at US$ 27.78 Bn in 2022 and is expected to exhibit a CAGR of 8.0% over the forecast period (2022-2030)
Figure 1: Global HPAPIs and Cytotoxic Drugs Manufacturing Market Share, (%), Analysis, By Drug Origin, 2022
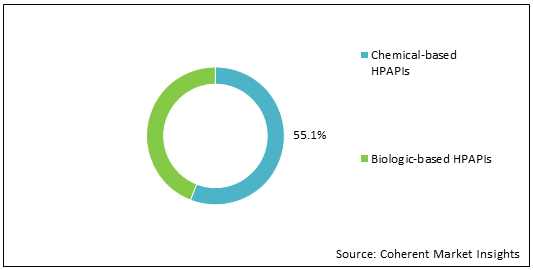
To learn more about this report, Download Free Sample
Market players are indulged in inorganic activities such as collaboration, in order to enhance company’s product portfolio. This is expected to drive growth of the global HPAPIs and cytotoxic drugs manufacturing market over the forecast period.
Market players are engaged in the inorganic activities such as collaborations and this is expected to drive growth of the global HPAPIs and cytotoxic drugs manufacturing market over the forecast period. In April 2020, Trio Pharmaceuticals, Inc. and Ajinomoto Bio-Pharma Services announced a collaboration agreement to evaluate AJICAP, a proprietary site-specific conjugation technology offered by Aji Bio-Pharma for the development of TDCs (TRIObody Drug Conjugate). The AJICAP technology will be used to conjugate a cytotoxic payload to TRIO’s lead oncology candidate with TRIO evaluating functionality of the TDC.
Moreover, in March 2021, SEQENS, a company integrated in pharmaceutical synthesis and specialty ingredients announced a collaboration with RONDOL, a technology leader in the development and refinement of medication dosage extrusion technologies for formulation research and continuous manufacturing for more effective therapies and more efficient supply chains. The overall goal was to speed up the development and commercialization of extruded dosage forms with greater bioavailability, fewer adverse effects, and lower production costs.
HPAPIs and Cytotoxic Drugs Manufacturing Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2021 | Market Size in 2022: | US$ 27.78 Bn |
| Historical Data for: | 2018 to 2020 | Forecast Period: | 2022 to 2030 |
| Forecast Period 2022 to 2030 CAGR: | 8.0% | 2030 Value Projection: | US$ 51.53 Bn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Teva Pharmaceutical Industries Ltd., Pfizer, Inc., Lonza Group, CordenPharma International, Evonik Industries AG, Flamma Group, Merck KGaA, CARBOGEN AMCIS, Catalent, Inc., Piramal Enterprises Ltd., AbbVie Inc., Fareva Group, Cerbios-Pharma SA, Novasep, Ajinomoto Bio-Pharma, PCI Pharma Services, Sterling Pharma Solutions, Heraeus Holding, Polpharma Biologics, Helsinn Healthcare SA, Seqens, Cambrex Corporation |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Market players are indulged in expansion of new manufacturing facility and this is expected to drive the growth of the global HPAPIs and cytotoxic drugs manufacturing market over the forecast period
Expansion of manufacturing facilities by the market players is expected to drive growth of the global HPAPIs and cytotoxic drugs manufacturing market over the forecast period. For instance, on March 21, 2022, SEQENS announced a multi-million dollar investment in Devens, Massachusetts-based research and development lab. Seqens' capabilities in the development and production of active pharmaceutical ingredients (APIs) and active delivery systems (lipids and polymers) will be enhanced by the new facility. The project is expected to be completed in 2023
Moreover, in October 2021, Cambrex announced that its manufacturing center of excellence in High Point, North Carolina, will be expanded. The investment of more than US$ 30 million is intended to fulfil the growing demand for small molecule development and production services. New chemical laboratories, two new clinical manufacturing sites, and a small-scale commercial manufacturing operation with three work centers and 2,000 liter reactors will also be part of the expansion.
Global HPAPIs and Cytotoxic Drugs Manufacturing Market – Restraints
Stringent regulatory scenario against HPAPIs and manufacturing of the cytotoxic drugs is expected to hamper the growth of the global HPAPIs and cytotoxic drugs manufacturing market, over the forecast period. Strict regulatory guidelines such as quality assessments of high potent active pharmaceutical ingredients and cytotoxic drug manufacturing, facility certification, enhanced cGMP guidelines tightening surprise inspection regimes, and increased supply chain security issues across emerging countries have a large impact on the cost of final products.
Due to these strict guidelines, the new entrants willing to enter into active pharmaceutical ingredient manufacturing are facing financial challenges, owing to the aforementioned factors. This makes the HPAPIs and cytotoxic drugs manufacturing entry a financially unprofitable for small and medium-sized enterprises (SMEs). Such changing regulatory dynamics are hampering the growth of the HPAPIs and cytotoxic drugs manufacturing market in emerging economies.
Global HPAPIs and Cytotoxic Drugs Manufacturing Market – Regional Analysis
On the basis of region, global HPAPIs and cytotoxic drugs manufacturing market is segmented into North America, Latin America, Europe, Asia Pacific, Middle East, and Africa
Among regions, North America HPAPIs and cytotoxic drugs manufacturing market is expected to hold a dominant position during the forecast period, owing to the increasing number of product approvals in the region. For instance, in 2019, Pfizer Inc. received approval from the U.S. Food and Drug Administration (FDA) for RUXIENCE (rituximab-pvvr), a biosimilar to Rituxan (rituximab) which is indicated for the treatment of adult patients with non-Hodgkin’s lymphoma (NHL), chronic lymphocytic leukemia (CLL), and granulomatosis with polyangiitis (GPA) and microscopic polyangiitis (MPA).
Furthermore, Europe is also estimated to witness significant growth in the global HPAPIs and cytotoxic drugs manufacturing market, owing to expansion of the HPAPI manufacturing facility in the region. For instance, in May 2021, Novasep, a leading provider of services and solutions to the life sciences industry, announced that its manufacturing capabilities for Highly Potent Active Pharmaceutical Ingredients (HPAPIs) had been expanded at its Le Mans (72 – France) site. Novasep's position as a leading CDMO for the manufacturing of innovative and tailored medicines to treat cancer is strengthened by this new step.
Figure 2: Global HPAPIs and Cytotoxic Drugs Manufacturing Market (US$ Bn), by Region, 2022
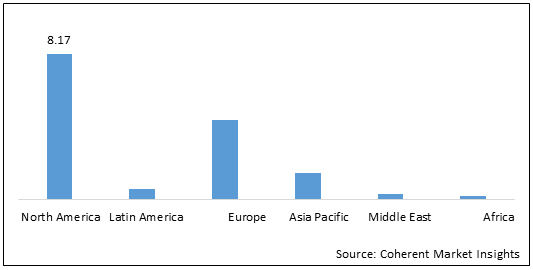
To learn more about this report, Download Free Sample
Global HPAPIs and Cytotoxic Drugs Manufacturing Market – Competitive Landscape
Major players operating in the global HPAPIs and cytotoxic drugs manufacturing market include Teva Pharmaceutical Industries Ltd., Pfizer, Inc., Lonza Group, CordenPharma International, Evonik Industries AG, Flamma Group, Merck KGaA, CARBOGEN AMCIS, Catalent, Inc., Piramal Enterprises Ltd., AbbVie Inc., Fareva Group, Cerbios-Pharma SA, Novasep, Ajinomoto Bio-Pharma, PCI Pharma Services, Sterling Pharma Solutions, Heraeus Holding, Polpharma Biologics, Helsinn Healthcare SA, Seqens, Cambrex Corporation.
Share
Share
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients