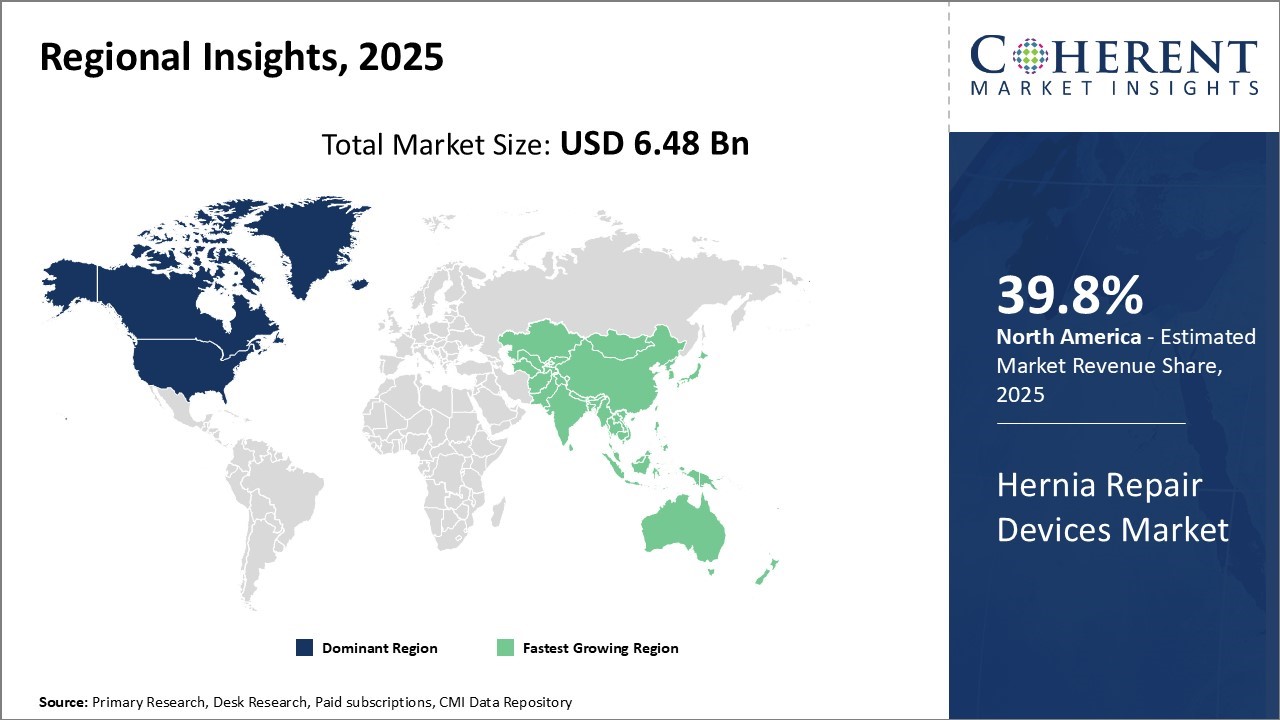
The Global Hernia Repair Devices Market is estimated to be valued at USD 6.48 Bn in 2025 and is expected to reach USD 8.7 Bn by 2032, exhibiting a compound annual growth rate (CAGR) of 4.3% from 2025 to 2032.
The global market for hernia repair devices is witnessing consistent growth with the rising incidence of hernias and the preference for minimally invasive procedures. The growth in the aging population globally, particularly both in developed as well as developing economies, continues to be a key driver since the prevalence of hernia increases with age as well as associated conditions like obesity and weakened abdominal walls. Sustained business expansion is being propelled by technology developments in surgical mesh technologies—synthetic and biologic—improving patients' recovery times and decreasing the rates of recurrence.
|
Current Events |
Description and its impact |
|
Push Towards Light Weight, Biologic & Sustainable Meshes
|
|
|
Integration of Robotic-Assisted and AI-Guided Hernia Repairs
|
|
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
To learn more about this report, Download Free Sample
The prevalence of hernia cases has been steadily rising around the world in the past few decades. Hernias can occur at any age but the risk generally increases with age. With improved global life expectancy over the years, the population of aged people across the globe has risen considerably. The aged are more vulnerable to hernias because the muscles and tissues gradually weaken with age as part of the natural aging process.
Further, lifestyle aspects such as obesity have also played a role in the increased prevalence of hernias. With too much weight, intra-abdominal pressure increases that can stretch and weaken the abdominal wall to result in out-pouching of tissues or organs. According to Medscape's March 2023 report, over 1 million wall hernia repair operations are conducted annually within the U.S., with inguinal hernia repairs constituting about 770,000 of them. Around 90% of all inguinal hernia repairs are performed on men.
One of the major opportunities in the global hernia repair devices market is the growing demand for minimally invasive surgical procedures. There is an increasing preference among patients and surgeons for less invasive techniques like laparoscopic and robot-assisted hernia repair surgeries.
Minimally invasive procedures are associated with reduced post-operative pain, lower risk of surgical site infections control , shorter hospital stay, and quicker recovery time compared to open surgeries. This has driven the demand for single-incision laparoscopic systems, robotic surgical platforms, and other minimal access devices for hernia treatment. Furthermore, minimally invasive hernia repair allows patients to return to normal activities in a short time frame.
In terms of product, the mesh segment is expected to contribute the highest share of the market with 50.7% in 2025 owing to its proven clinical efficacy and biocompatibility. Mesh offers superior tissue integration and durability compared to other options like sutures and glues. Made from synthetic or biological materials like polypropylene, polyester, and porcine or bovine collagen, mesh implants provide a strong yet flexible reinforcement to securely close hernia defects.
Their porous structure allows tissue ingrowth for long-lasting hernia repair with minimal risk of recurrence. Synthetic meshes are preferred in most cases as they provoke minimal inflammatory response and exhibit optimal tensile strength over time. Absorbable meshes minimize complications associated with permanent implants while still allowing proper tissue remodeling.
In terms of procedure, the open surgery segment is expected to contribute the highest share of the market with 53.8% in 2025 owing to its familiarity among surgeons and relative technical simplicity. The open hernia repair technique has been used for decades and remains the procedure of choice in many healthcare settings globally. It allows for direct visualization and manipulation of tissues, permits use of different hernia mesh types, and provides opportunities for inspection of abdominal organs. Minimal special skills or advanced instruments are required to perform open surgeries.
Meanwhile, laparoscopic hernia repair is a minimally invasive alternative but requires specialized training, long learning curves, costly equipment, and technical expertise to operate miniature instruments within the abdominal cavity under endoscopic camera guidance.
In terms of clinical indication, the inguinal hernia segment is expected to contribute the highest share of the market with 35.7% in 2025 owing to its high prevalence and potential risks if left untreated. Epidemiological data indicates inguinal hernias account for over 75% of hernia cases globally with highest rates among adult men.
Located in the groin region where abdominal muscles are weak, inguinal hernias frequently develop from normal day-to-day activities that increase intra-abdominal pressure like coughing, lifting, and straining. While small hernias may be asymptomatic, larger defects can cause pain, discomfort, /bowel obstruction, and reduced quality of life if incarcerated or strangulated. Prompt surgical correction is thus usually advised to avoid potential complications.
To learn more about this report, Download Free Sample
In North America, the dominance in the hernia repair devices market with a share of 39.8% in 2025 can be attributed to factors such as the strong presence of key players, high healthcare expenditure, rapid adoption of advanced medical technologies, and favorable reimbursement policies. For instance, leading companies like Becton, Dickinson and Company (BD), and Johnson & Johnson have established manufacturing bases and supply networks across the region.
Meanwhile, the Asia Pacific region exhibits the fastest growth with a share of 26% in 2025 aided by rising medical tourism, growing general population, increasing healthcare spending, and government initiatives to modernize healthcare infrastructure in emerging economies. Countries like China, India, and South Korea are attractive destinations for major players looking to tap into high-potential markets.
The U.S. hernia repair devices market is dominated by notable companies focusing on new product development and acquisitions to strengthen their market positions. For example, major players are collaborating with surgeons to design clinically-validated devices. According to the National Center for Biotechnology Information, as of March 2023, the U.S. has a high prevalence of ventral and incisional hernias. Ventral hernias affect approximately 20.5% of adults, while incisional hernias occur in up to 30.5% of individuals with midline abdominal incisions.
In March 2023, according to the National Center for Biotechnology Information, over 800,000 Incisional and Ventral Hernia Repair (IVHR) surgeries were performed in Australia. This marks a significant rise in the number of IVHR surgeries in the past 20 years, especially for primary ventral hernias.
The hernia repair devices market in India is expanding due to rising awareness and access to healthcare. In December 2023, the Indian government announced funding for surgical training programs, which will improve the skills of healthcare professionals in hernia repair techniques.
There is high growth for China in the hernia repair devices market, owing to growing healthcare spending and an expanding patient base. In March 2024, the Chinese government initiated a program, Surgical Care Advancement Program, to improve surgical care, including encouraging innovative hernia repair technologies.
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 6.48 Bn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 4.3% | 2032 Value Projection: | USD 8.7 Bn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
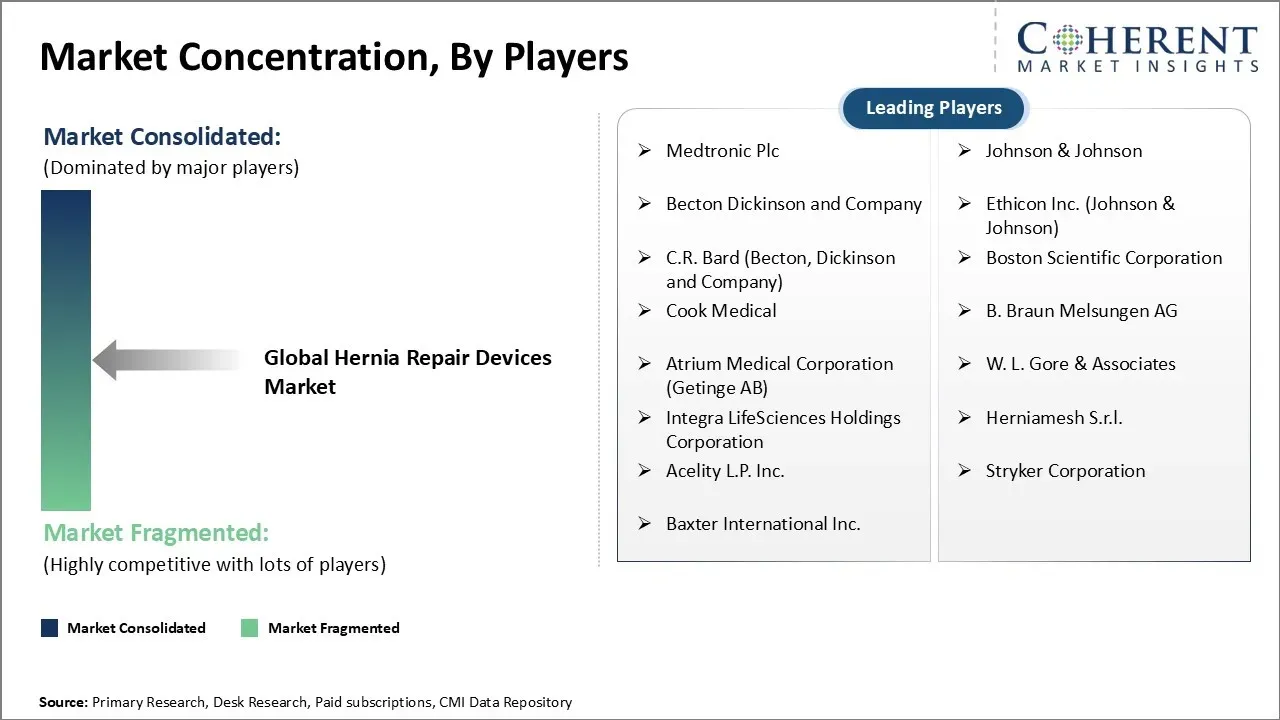
Medtronic Plc, Johnson & Johnson, Becton Dickinson and Company, Ethicon Inc. (Johnson & Johnson), C.R. Bard (Becton, Dickinson and Company), Boston Scientific Corporation, Cook Medical, B. Braun Melsungen AG, Atrium Medical Corporation (Getinge AB), W. L. Gore & Associates, Integra LifeSciences Holdings Corporation, Herniamesh S.r.l., Acelity L.P. Inc., Stryker Corporation, and Baxter International Inc. |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Share
Share
About Author
Manisha Vibhute is a consultant with over 5 years of experience in market research and consulting. With a strong understanding of market dynamics, Manisha assists clients in developing effective market access strategies. She helps medical device companies navigate pricing, reimbursement, and regulatory pathways to ensure successful product launches.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients