Hernia Mesh Devices Market Size and Forecast – 2025 – 2032
The Global Hernia Mesh Devices Market size is estimated to be valued at USD 1.8 billion in 2025 and is expected to reach USD 3.5 billion by 2032, exhibiting a compound annual growth rate (CAGR) of 9.3% from 2025 to 2032.
Global Hernia Mesh Devices Market Overview
Hernia mesh devices are implantable surgical products designed to reinforce weakened or damaged tissue in hernia repair procedures. Available in synthetic, biological, and composite materials, these products are engineered to provide strength, flexibility, and biocompatibility. Mesh devices may feature absorbable or non-absorbable designs and are available in various shapes and sizes to suit specific hernia types, offering surgeons options for laparoscopic or open repair techniques.
Key Takeaways
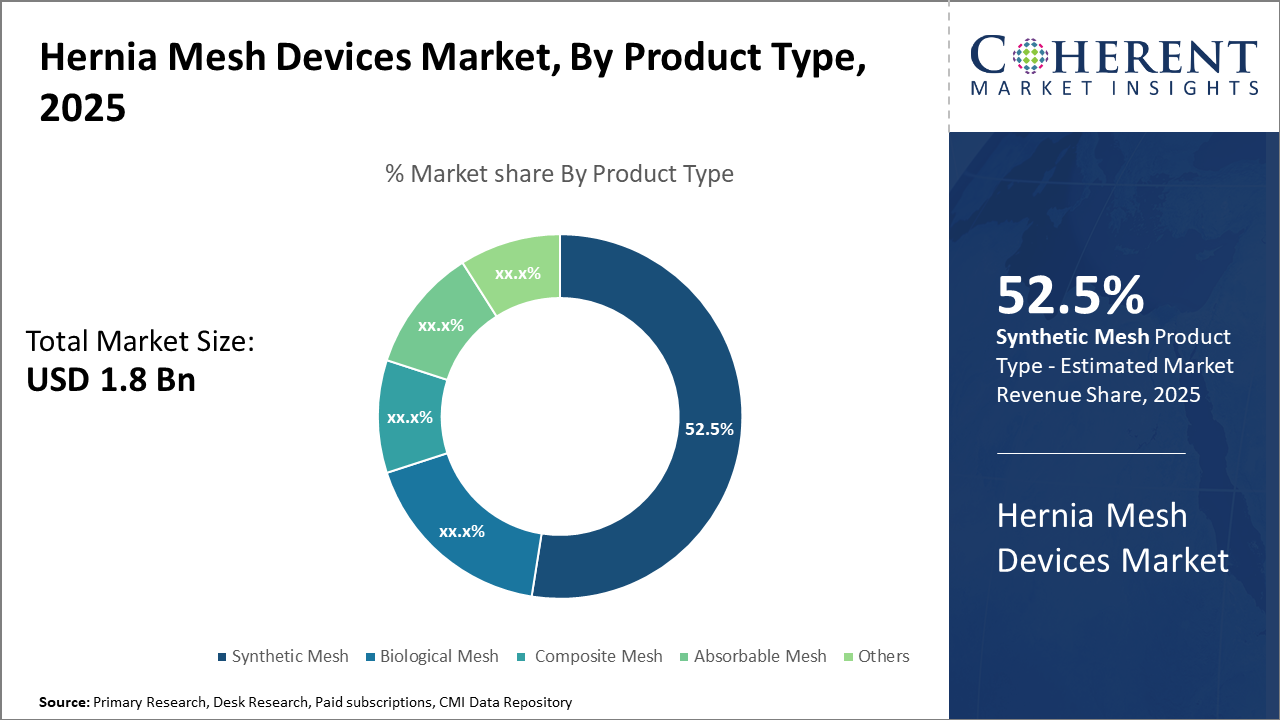
In the product segment, synthetic mesh dominates 52.5% of the market share due to its cost efficiency and reliability, while biological mesh is gaining traction in specialized applications emphasizing biocompatibility.
The laparoscopic surgery segment emerges as the fastest-growing surgery type due to minimally invasive benefits and expanding surgeon expertise, especially in developed nations.
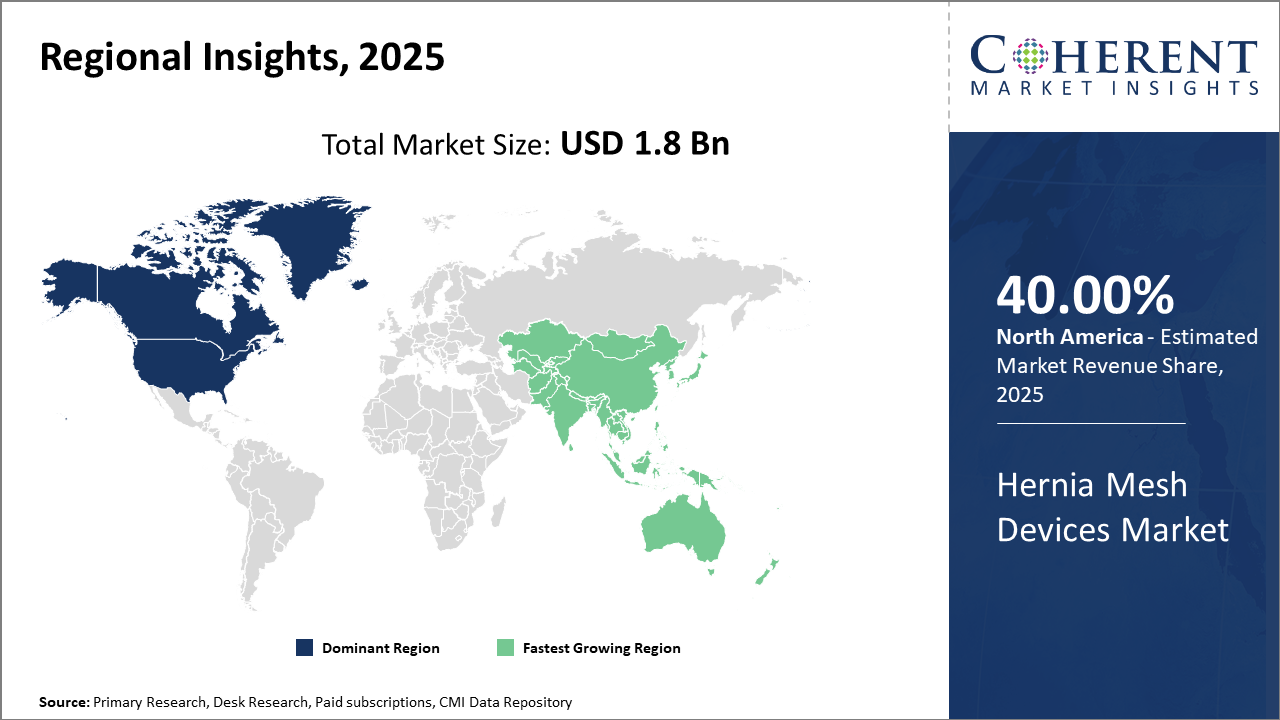
North America leads the regional market thanks to advanced healthcare infrastructure, accounting for 40% industry share, driven by innovations and reimbursement ecosystems.
Asia Pacific is the fastest-growing region, with a CAGR exceeding 11% propelled by increasing medical tourism and government policies expanding surgical access.
Hernia Mesh Devices Market Segmentation Analysis
To learn more about this report, Download Free Sample
Hernia Mesh Devices Market Insights, By Product Type
Synthetic Mesh dominates the market share at 52.5%. The dominance of synthetic mesh is primarily due to its affordability, versatility, and long-standing clinical efficacy, making it the preferred choice in most hernia repairs globally. Synthetic meshes, commonly made of polypropylene, have proven durability and are widely used in open, laparoscopic, and robotic surgeries, contributing to large-scale market revenue. Biological mesh is emerging as a preferred option for contaminated or complex hernia cases due to its superior biocompatibility and reduced risk of infection, making it the fastest growing subsegment as surgeons seek more patient-specific solutions.
Hernia Mesh Devices Market Insights, By Application
Inguinal Hernia dominates this segment owing to its highest prevalence globally, accounting for over 40% of all hernia surgeries. Its high incidence amongst aging and obese populations results in consistent clinical demand for mesh implants designed specifically for this application. Incisional Hernia, although lower in overall volume, is the fastest growing subsegment due to increased post-surgical hernia complications, especially in laparotomy cases. This has augmented the utilization of advanced mesh products designed for stronger tissue reinforcement.
Hernia Mesh Devices Market Insights, By Surgery Type
Open surgery holds a traditional and stable presence; however, laparoscopic surgery leads as the fastest-growing segment propelled by minimally invasive procedure benefits such as faster recovery and reduced infection risk. Clinical data suggest laparoscopic mesh repair procedures increased by 25% between 2023 and 2025 globally, driven by surgeon adoption and patient preference. Robotic surgery, while a relatively newer segment, exhibits rapid growth, especially in North America and Europe where cutting-edge medical centers have integrated robotic platforms, providing enhanced precision in mesh placement.
Hernia Mesh Devices Market Trends
The market is witnessing a convergence of technological advancements with clinical demand driving innovation adoption.
Notably, antimicrobial and bioabsorbable mesh materials are on the rise due to their ability to reduce postoperative infection rates, confirmed by 2025 case studies showing up to 30% fewer complications when used.
The escalation in robotic-assisted hernia repairs highlights the integration of precision instruments in surgery, pushing demand for specialized mesh products.
Another notable development is the incorporation of AI in patient selection and preoperative evaluation, optimizing device choice outcomes and contributing to market reliability and growth.
Hernia Mesh Devices Market Insights, By Geography
To learn more about this report, Download Free Sample
North America Hernia Mesh Devices Market Analysis and Trends
In North America, the dominance in the Hernia Mesh Devices market stems from expansive healthcare infrastructure, significant R&D investments, and favorable reimbursement frameworks. The region accounts for more than 40% of industry share primarily due to high procedural volumes in the U.S. and Canada. The presence of major market companies like Johnson & Johnson and Medtronic contributes to technological leadership and rapid adoption of novel mesh products. Government initiatives promoting minimally invasive surgeries have further accelerated market penetration.
Asia Pacific Hernia Mesh Devices Market Analysis and Trends
Meanwhile, the Asia Pacific exhibits the fastest growth with a CAGR exceeding 11%, driven by rising healthcare accessibility, increasing prevalence of hernia cases, and rapidly expanding surgical infrastructure in countries like China and India. Government healthcare reforms and expanding medical tourism enhance demand. Local and international companies actively invest in market expansion strategies, collaborating with regional hospitals to introduce advanced hernia mesh devices. Growing awareness and affordability contribute heavily to this upward trend.
Hernia Mesh Devices Market Outlook for Key Countries
USA Hernia Mesh Devices Market Analysis and Trends
The USA's Hernia Mesh Devices market holds a critical position globally due to high procedural volumes, advanced surgical infrastructure, and significant investments in healthcare innovation. In 2024, the U.S. reported over 1 million hernia repairs annually, with a steady increase in robotic and laparoscopic surgeries. Leading companies such as Johnson & Johnson and Medtronic hold substantial shares, introducing cutting-edge composite meshes and bioengineered products. The country’s well-established reimbursement framework and strong clinical research landscape further stimulate market revenue and business growth opportunities.
Germany Hernia Mesh Devices Market Analysis and Trends
Germany's hernia mesh devices market demonstrates consistent growth fueled by a robust healthcare system, high public health expenditure, and early adoption of innovative surgical techniques. In 2025, hospitals across Germany accounted for nearly 12% of the European volume in hernia repairs, predominantly favoring synthetic and composite meshes. Key players like B. Braun have leveraged Germany’s R&D capabilities to introduce novel lightweight mesh designs focused on patient comfort and reduced complications. This progress is complemented by government policies supporting medical device innovation and quality standards, making Germany a significant contributor to the European industry revenue.
Analyst Opinion
Increasing preference for synthetic meshes over biological counterparts significantly influences the hernia mesh devices market share. Recent hospital procurement data from 2024 highlights that approximately 68% of hernia surgeries worldwide utilized synthetic meshes due to their cost-effectiveness and durability. This shift underscores a demand-side indicator that propels market revenue growth substantially.
Supply-side dynamics reveal enhancements in manufacturing capacities of advanced mesh materials such as polypropylene and polyester. Production output in 2025 saw a 15% increase compared to 2023, largely due to investments in automation and stringent quality controls across leading manufacturing hubs, indicating strong supply reliability for market players.
Emerging use cases in laparoscopic and robotic hernia repair have opened new growth avenues. Clinical registries document a 27% year-on-year increase in such procedures in North America and Europe in 2024, directly translating to increased imports and supply of specialized hernia mesh devices tailored for these applications.
Pricing trends have demonstrated relative stability despite raw material costs fluctuations, supported by volume-driven economies in production. Recent pricing indexes from 2025 suggest an average price range for standard mesh devices has remained within a 3% variation annually, facilitating broader adoption across emerging markets.
Market Scope
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2025 | Market Size in 2025: | USD 1.8 billion |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 9.3% | 2032 Value Projection: | USD 3.5 billion |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: | Johnson & Johnson, Medtronic, B. Braun Melsungen AG, C.R. Bard (now part of BD), Terumo Corporation, Baxter International Inc., Integra LifeSciences, Biom’up, Surgical Innovations Group PLC, Smith & Nephew, Becton, Dickinson and Company | ||
| Growth Drivers: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Hernia Mesh Devices Market Growth Factors
Major drivers fueling the hernia mesh devices market growth include the rising hernia incidence attributed to aging populations and obesity, which has led to a tangible increase in surgical interventions globally. For instance, CDC data from 2024 reflects a 6% annual increase in hernia repair surgeries in the U.S. The growing adoption of minimally invasive surgical techniques such as laparoscopic and robotic repairs is another catalyst, backed by clinical trials indicating reduced recovery time and complication rates, driving surgeon preference and instrument demand.
Technological advancements in mesh materials—especially in lightweight, composite, and bioabsorbable meshes—support enhanced patient outcomes and expanded clinical indications. Regulatory approvals of innovative devices have accelerated market opportunities particularly in Europe and Asia Pacific. Lastly, expanding healthcare access in emerging economies, supported by government initiatives for infrastructural development, is broadening the market scope in regions such as India and Brazil.
Hernia Mesh Devices Market Development
In April 2025, Becton, Dickinson and Company (BD) introduced the Phasix™ ST Umbilical Hernia Patch following U.S. FDA 510(k) clearance, marking the launch of the first bioabsorbable mesh specifically engineered for umbilical hernia repair. The patch is composed of poly-4-hydroxybutyrate (P4HB), a fully resorbable polymer that provides the necessary strength for abdominal wall reinforcement while gradually being absorbed by the body as tissue healing occurs.
In April 2024, Deep Blue Medical Advances expanded its hernia repair portfolio with the launch of the T-Line® Mini Mesh, an innovative device designed for the repair of small umbilical and epigastric hernias. The T-Line Mini incorporates the company’s patented T-Line tension-distribution technology, which extends load-bearing fibers beyond the edge of the mesh to create a stronger, more secure fixation without relying heavily on sutures or tacks. This design minimizes stress concentration at the repair site, reducing recurrence risk and improving patient comfort.
Key Players
Leading companies of the market:
Johnson & Johnson
Medtronic
B. Braun Melsungen AG
C.R. Bard (now part of BD)
Terumo Corporation
Integra LifeSciences
Biom’up
Surgical Innovations Group PLC
Smith & Nephew
Becton, Dickinson and Company
A notable market strategy includes Johnson & Johnson launching a bioengineered mesh in 2025, enhancing postoperative tissue integration, and recording a 12% increase in sales revenue within six months. Similarly, Medtronic’s strategic acquisition of smaller niche device companies in 2024 allowed expansion into robotic surgery mesh solutions, resulting in a 20% market share uplift in North America.
Hernia Mesh Devices Market Future Outlook
The market is projected to expand with continued innovation in material science, minimally invasive procedures, and patient-specific solutions. Absorbable and hybrid meshes, designed for enhanced tissue integration and reduced postoperative complications, are expected to drive adoption. Growing awareness of mesh safety, coupled with ongoing product development and improved regulatory pathways, will support sustainable market growth. Additionally, increasing outpatient surgical procedures and technological integration with robotic-assisted surgery will create new opportunities for market expansion globally.
Hernia Mesh Devices Market Historical Analysis
The hernia mesh devices market has a long history rooted in surgical innovations aimed at reducing recurrence and improving repair outcomes. Early hernia repairs relied on suture-based techniques, which often resulted in high recurrence rates. The introduction of synthetic and biological mesh materials revolutionized the market, providing durable reinforcement for abdominal walls and enabling both open and minimally invasive procedures. Over time, advancements in biocompatibility, absorbable composites, and customizable designs allowed surgeons to select mesh types suited for specific patient needs and hernia types. Regulatory standards, clinical studies, and increased surgeon training further contributed to widespread adoption.
Sources
Primary Research interviews:
General Surgeons
Biomedical Engineers
Operating Room Managers
Medical Device Manufacturers
Product Design Specialists
Databases:
FDA MAUDE Database
PubMed
GlobalData Medical Devices
Magazines:
Medical Design & Outsourcing
MedTech Dive
Surgical Products Magazine
Medical Device Network
Journals:
Surgical Endoscopy
Journal of Biomedical Materials Research
Journal of the American College of Surgeons
Newspapers:
The New York Times (Health)
The Guardian (Science)
The Washington Post (Health)
The Economic Times (Healthcare)
Associations:
American Hernia Society (AHS)
Society of American Gastrointestinal and Endoscopic Surgeons (SAGES)
U.S. FDA
European Hernia Society
Share
Share
About Author
Manisha Vibhute is a consultant with over 5 years of experience in market research and consulting. With a strong understanding of market dynamics, Manisha assists clients in developing effective market access strategies. She helps medical device companies navigate pricing, reimbursement, and regulatory pathways to ensure successful product launches.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients