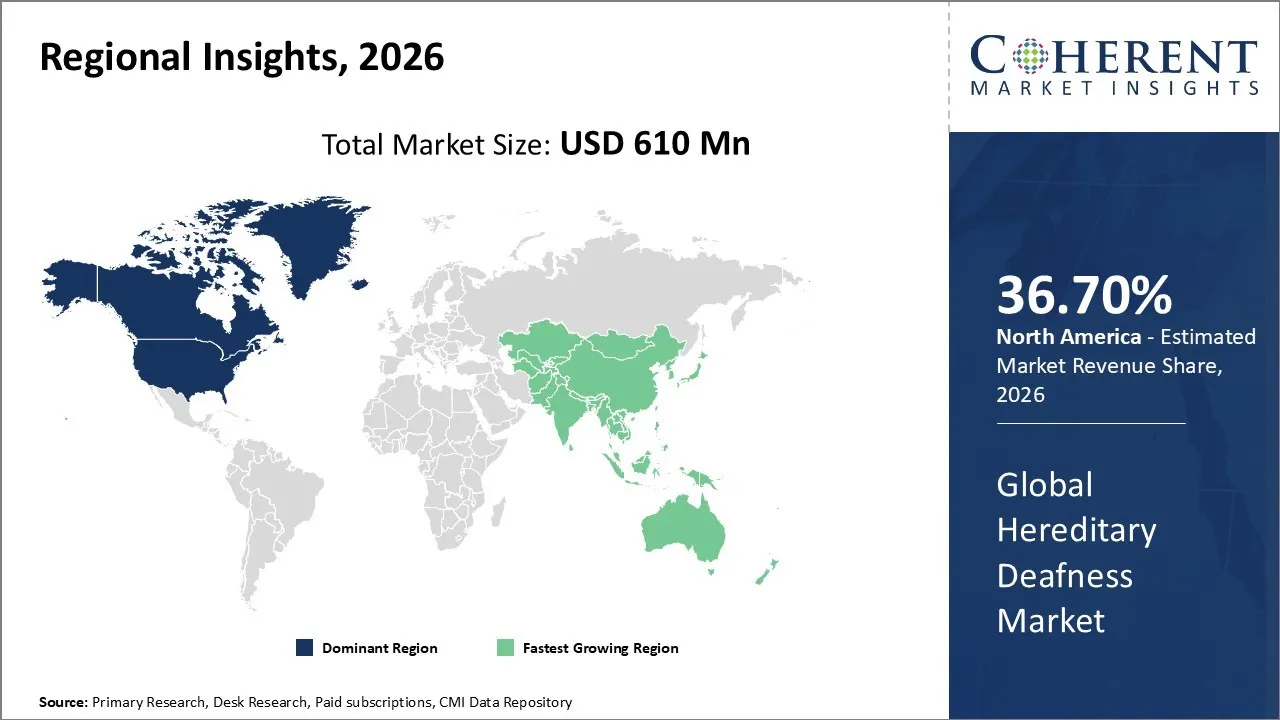
The Hereditary Deafness Market is estimated to be valued at USD 610 Mn in 2026 and is expected to reach USD 1,045 Mn by 2033, growing at a compound annual growth rate (CAGR) of 8% from 2026 to 2033.
The Hereditary Deafness Market provides advanced audiological diagnostic systems and therapeutic technologies primarily for infants and individuals with genetic hearing impairments. The market includes sophisticated cochlear implants, bone-anchored hearing systems, and digital hearing aids, along with associated genetic testing kits, surgical equipment, and long-term rehabilitation services.
The global prevalence of congenital hearing loss is being identified more frequently each year, thus creating a greater demand for early intervention systems among neonatal centers and specialized audiology clinics. Healthcare providers and families recognize the critical importance of early diagnosis for speed development and long-term public health outcomes. Government environmental agencies now require universal newborn hearing screening, making molecular genetic testing and assistive hearing devices a standard requirement in pediatric healthcare operations.
|
Current Event |
Description and the Impact |
|
Advances in Gene Editing Technologies
|
|
|
Increasing Genetic Screening Programs
|
|
|
Technological Integration in Clinical Management |
|
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
In terms of Product Type, the Hearing Aids segment contributes the highest share of 68.70% in 2026 of the market. This is because these devices have always remained easily accessible and noninvasive to patients. Also, these have remained one of the basic forms of treatment for patients experiencing genetic hearing impairment in its mild to moderate forms. Advancements in technology have largely helped this market grow with innovations such as AI processors, rechargeable lithium-ion batteries, and wireless connectivity, which easily pairs with smartphones. Also, over-the-counter hearing aids have made it easy for patients to access these, which have become a cost-efficient and conventional first-line treatment option across the globe.
For instance, in August 2024, Beltone, a leading US hearing care provider, introduced the pioneering Auracast™ broadcast audio portable microphone via its new Beltone Multi-Mic+ alongside updated Auracast-compatible versions of the Beltone Serene™ hearing aids. Tailored to integrate effortlessly with Beltone Serene devices, the Multi-Mic+ assists those with hearing challenges in crowded settings by boosting speech from single or multiple speakers while preserving awareness of surrounding noises.
In terms of end user, the hospitals segment contributes the highest share of 44.55% in 2026 of the market. This is because hospitals act as the main center for the team-based care needed for genetic conditions that range from newborn screening to complex surgeries. Most cochlear implant and bone-anchored hearing surgeries take place in these settings due to the requirement for specialized operating rooms and close care after surgery. They have the advanced genetics labs needed to find specific hearing loss mutations, so the hospital remains the main setting of diagnosis, surgery, and long-term recovery support.
To learn more about this report, Download Free Sample
North America has remained the dominant region with 36.70% in 2026 of the global Hereditary Deafness Market over the past decade. The growth is owing to rising awareness, advanced technology, and robust healthcare infrastructure. The supportive policies, including strong regulatory support from the FDA and reimbursement policies in the region, enhance accessibility.
The market is shifting from palliative sound amplification toward permanent biological restoration. This transition is owing to the implementation of genetic diagnostics into neonatal infant care. This structural change centers on identifying monogenic triggers, specifically mutations in the GJB2 and OTOF genes, thus facilitating precision interventions such as adeno-associated virus-mediated gene delivery and CRISPR-based nerve repair.
The clinical landscape is defined by an acceleration in gene therapy trials and the use of digital health tools to optimize the performance of cochlear and bone-anchored systems. The market growth is characterized by strategic appointments between medical manufacturers and biopharmaceutical developers, which aims to unify base solutions with biological recovery.
For instance, in May 2025, Rznomics Inc., a biopharma company focused on RNA therapeutics from South Korea, revealed a global research partnership and licensing deal with Eli Lilly and Company. They will work together for the creation and market rollout of innovative RNA-editing treatments based on Rznomics' unique trans-splicing ribozyme technology, with a greater focus on therapies for sensorineural hearing loss.
Asia Pacific is the fastest-growing market, fueled by a rising focus on early intervention and neonatal care. As healthcare infrastructure strengthens in emerging economies, genetic screening gains prominence for detecting hereditary hearing loss at birth. This growth is can be attributed to the rising public awareness and government initiatives aimed at reducing the social and economic impact of untreated impairment. Families are seeking cutting-edge diagnostic services, which has encouraged global medical technology providers to strengthen their presence through localized manufacturing and distribution partnerships.
Innovations in assistive technology and regenerative medicine are reshaping the regional landscape. This is due to the surging demand for cutting-edge hearing solutions like cochlear implants and digital hearing aids. The region has become a hub for research in gene therapies, with numerous clinical trials exploring ways to address the root causes of genetic deafness. The overall market continues to evolve as digital health tools and telehealth services expand their access to specialized audiology care.
For instance, in February 2024, the Avestagenome Project® International Pvt. Ltd. and Avesthagen Limited teamed up with the Tata Institute for Genetics and Society (TIGS) through a Strategic Alliance Agreement. The collaboration aims to speed research and development for rare genetic disorders, with a special focus on the Zoroastrian Parsi population. It targets conditions such as congenital deafness, muscular dystrophies, Parkinson’s disease, multiple sclerosis, and other understudied illnesses to enable the development of new treatments and health solutions.
The U.S. market is characterized by a high-quality system for early medical help and a strong line of new biological inventions for patients. The country uses universal baby screening programs that help doctors identify genetic hearing loss shortly after a child is born. This practice testing environment has boosted the use of modern hearing tech, including new cochlear implants and advanced systems that send sound through the bone.
Currently the market is moving toward personalized care, with any new test for gene-fixing treatments and ways to regrow damaged cells in the inner ear. High spending on health and helpful laws for rare diseases continue to bring in big medical companies that want to sell new hearing products. These factors make the US a main center for selling permanent cures rather than just tools that help people with their hearing loss.
For instance, in January 2024, the Children's Hospital of Philadelphia (CHOP), for example, revealed the first outcomes of an experimental gene therapy for a patient with hereditary hearing loss in the US. The initial results suggest that the treatment could be effective.
China's market for hereditary hearing loss is characterized by its vast patient population and a rapidly evolving government strategy to enhance public health outcomes. Since genes cause many cases of birth-related hearing loss, the government now focuses on massive programs that test for both hearing and genetic markers. While common help like hearing aids and hearing implants is still standard, many people in the countryside still cannot get the medical care they need. However, China is becoming a top player in gene research, lately gaining attention for trials that fixed hearing in children using safe modified viruses.
For instance, in January 2024, the Eye and ENT Hospital of Fudan University, along with collaborators from Harvard Medical School, Southeast University, and Shanghai Dingxin Gene Technology Co., Ltd., published a groundbreaking clinical trial titled “AAV1-hOTOF Gene Therapy for Autosomal Recessive Deafness 9: a single-arm trial. This is the first successful gene therapy clinical trial for congenital hearing loss in history.
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2025 | Market Size in 2026: | USD 610 Mn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2026 To 2033 |
| Forecast Period 2026 to 2033 CAGR: | 8% | 2033 Value Projection: | USD 1,045 Mn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Cochlear Medical Device Company India Pvt Ltd, Envoy Medical Corporation, MED-EL GmbH, Advanced Bionics, Nurotron Biotechnology Inc., Oticon Medical, Medtronic, Sonova Hearing India Pvt., Audina Hearing Instruments Inc., RION Co., Ltd., GN Hearing, Decibel Therapeutics Inc., and Frequency Therapeutics, and Pipeline Therapeutics, Inc. |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
The increasing prevalence of inherited auditory conditions is the primary driver for the expansion of the hereditary deafness market. Genetic mutations, ranging from non-syndromic variants to complex syndromes, represent a substantial portion of hearing impairment cases globally. This widespread occurrence has necessitated a robust shift toward specialized diagnostic and therapeutic solutions. As more individuals are identified with hereditary predispositions, there is an intensified demand for early screening programs and precise genetic testing to determine the specific molecular cases of impairment.
The rising awareness among healthcare providers and patients regarding the hereditary nature of these conditions is speeding up the adoption of advanced interventions. This trend is fueling significant investments in gene therapy and regenerative medicine alongside the continual refinement of cochlear implants and digital hearing aids. By placing a greater focus on the biological roots of hearing loss, the market is evolving toward a personalized medicine approach. The consistent identification of new genetic markers and the increasing focus on neonatal screening ensure a steady pipeline of patients requiring long-term management, thereby sustaining momentum and driving continuous innovation in auditory care.
Nearly 2.5 billion people are expected to have some form of hearing loss by 2050, and over 700 million of them will need hearing rehabilitation, according to a World Health Organization report.
Definition: The Hereditary Deafness Market focuses on medical systems and therapeutic solutions that help treat hearing loss caused by genetic mutations. These diagnostic and treatment systems are widely used in specialized hospitals and audiology clinics where genetic screening is now a standard part of newborn and pediatric healthcare. Targeted genetic therapies and advanced cochlear implants limit the impact of sensorineural impairment and reduce the long-term developmental challenges linked to congenital deafness by restoring auditory function before children reach clinical language milestones. The demand for these solutions comes from neonatal units, research institutions, and biopharmaceutical companies, all of which must adhere to strict clinical and regulatory mandates.
Share
Share
About Author
Manisha Vibhute is a consultant with over 5 years of experience in market research and consulting. With a strong understanding of market dynamics, Manisha assists clients in developing effective market access strategies. She helps medical device companies navigate pricing, reimbursement, and regulatory pathways to ensure successful product launches.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients