Herceptin is a monoclonal antibody used to treat patients suffering from metastatic (spread) breast cancer and gastric cancers. Hereceptin is used as part of chemotherapy regimen for adjuvant treatment of lymph-node positive, HER2/neu protein positive breast cancer. Herceptin is also known as trastuzumab and mainly administered intravenously in cancer patients, the dose of herceptin given to patients is evaluated on the basis of patients' disease history and other physiological factors such as height, weight, type of cancer or disease condition. The first dose of Herceptin is administered intravenously for over 90 minutes, and if it is tolerated in patients then the maintenance dose is administered for 30 minutes.
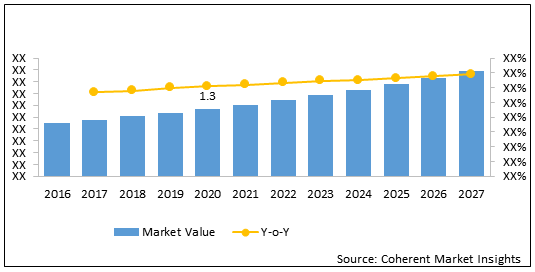
The global Herceptin biosimilar market size is estimated to be valued at US$ 1.3 billion in 2020 and is expected to exhibit a CAGR of 24.5 % over the forecast period (2020-2027).
Figure 1. Global Herceptin Biosimilar Market Value (US$ Bn), 2016-2017
To learn more about this report, Download Free Sample
The increasing prevalence of cancers such as breast cancer in women across the globe is a major factor driving the growth of the global herceptin biosimilar market. According to the World Health Organization’s report published in 2019, approximately 627,000 women across the globe died from breast cancer and it accounted for around 15% of all cancer deaths among women in 2018.
Furthermore, the increasing prevalence of gastric cancers such as metastatic gastric cancer are the factors expected to drive the growth of the global herceptin biosimilar market. According to the US National Library of Medicine’s report published in 2018, gastric cancer is the fifth most common neoplasm in people and third most deadly cancer across the globe. According to the same source, gastric cancer accounted for around 783,000 death globally in 2018.
Herceptin Biosimilar Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2019 | Market Size in 2019: | US$ 1.3 Bn |
| Historical Data for: | 2016 to 2019 | Forecast Period: | 2020 to 2027 |
| Forecast Period 2020 to 2027 CAGR: | 24.5 % | 2027 Value Projection: | US$ 5.8 Bn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Amgen Inc., AryoGen Biopharma, Biocon Limited, Celltrion Inc., Pfizer Inc., Merck & Co., Inc., Accord Healthcare Ltd, Gedeon Richter Plc, Genor Biopharma Company Ltd, Mabion SA, Mylan N.V, Roche Holding AG and Samsungbioepis Co,.Ltd. |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Global Herceptin Biosimilar Market – Impact of Coronavirus (COVID-19)
Following the outbreak of COVID-19 in December 2019, the disease has spread to over 100 countries across the globe and the World Health Organization has declared it a public health emergency. According to the World Health Organization’s report, the manifestation of the coronavirus (COVID-19) has resulted in more than 38 million infected individuals worldwide as of 16th October 2020.
COVID-19 has affected each and every market around the world and has also impacted the global herceptin biosimilar market in the following ways: by directly affecting the production and demand; by creating disruptions in distribution channels; and by its financial impact on firms and financial markets. Supply chain and manufacturing activities in India, China, and the U.S. have been disrupted due to nationwide lockdowns. While countries such as Saudi Arabia, UAE, Egypt, and others are facing problems with regards to transportation of drugs from one place to another.
Moreover, various key players operating in the global herceptin biosimilar market are estimated to witness a decline in sales of biosimilars due to the COVID-19 pandemic and lockdowns imposed by governments in countries such as India, China, U.S. For instance, in July 2020, Roche Holding AG, a leading manufacturing of oncology medicines, stated that Roche’s top three selling biosimilar, Avastin, Rituxan and Herceptin experienced drop in sales of nearly 39%, 33% and 46% respectively due to the COVID-19 pandemic. Moreover, Roche witnessed loss of US$ 2.3 billion in the first quarter of 2020.
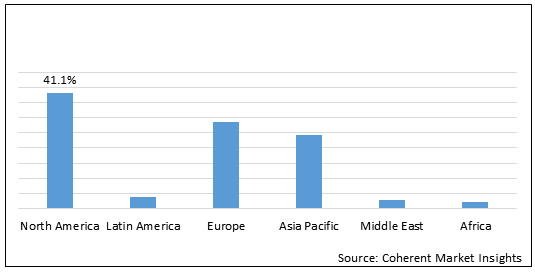
Figure 2. Global Herceptin Biosimilar Market Share (%), by Region, 2020
To learn more about this report, Download Free Sample
Among regions, North America is expected to witness significant growth in the global herceptin biosimilar market during forecast period, as companies focusing on obtaining approvals and launching products for treatment of cancers such breast and gastric cancer. For instance, in 2019, Amgen Inc. and Allergan plc. collaboratively launched KANJINTI (trastuzumab) for treatment of HER2-overexpressing adjuvant and metastatic breast cancer and HER2-overexpressing metastatic gastric or gastroesophageal junction adenocarcinoma. KANJINTI received approval from the U.S. Food Drug and Administration and can be used for treatment of breast cancer and gastric cancer throughout the U.S.
Key Players
Major players operating in the global herceptin biosimilar market include Amgen Inc., AryoGen Biopharma, Biocon Limited, Celltrion Inc., Pfizer Inc., Merck & Co., Inc., Accord Healthcare Ltd, Gedeon Richter Plc, Genor Biopharma Company Ltd, Mabion SA, Mylan N.V, Roche Holding AG and Samsungbioepis Co,.Ltd.
Share
Share
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients