Hepatocellular Carcinoma Drugs Market Size and Forecast – 2025 – 2032
The Global Hepatocellular Carcinoma Drugs Market size is estimated to be valued at USD 6.8 billion in 2025 and is expected to reach USD 11.5 billion by 2032, exhibiting a compound annual growth rate (CAGR) of 8.1% from 2025 to 2032.
Global Hepatocellular Carcinoma Drugs Market Overview
Hepatocellular Carcinoma drugs are specialized pharmacological products developed to manage primary liver cancer by inhibiting tumor growth, modulating the immune system, or inducing apoptosis in malignant cells. These products include small-molecule kinase inhibitors, checkpoint inhibitors, and combination regimens with localized therapies such as chemoembolization. Formulations are optimized for hepatic metabolism, oral or intravenous delivery, and patient adherence. Research is advancing toward biomarker-driven therapies that target specific molecular pathways, enabling personalized treatment approaches for better therapeutic outcomes.
Key Takeaways
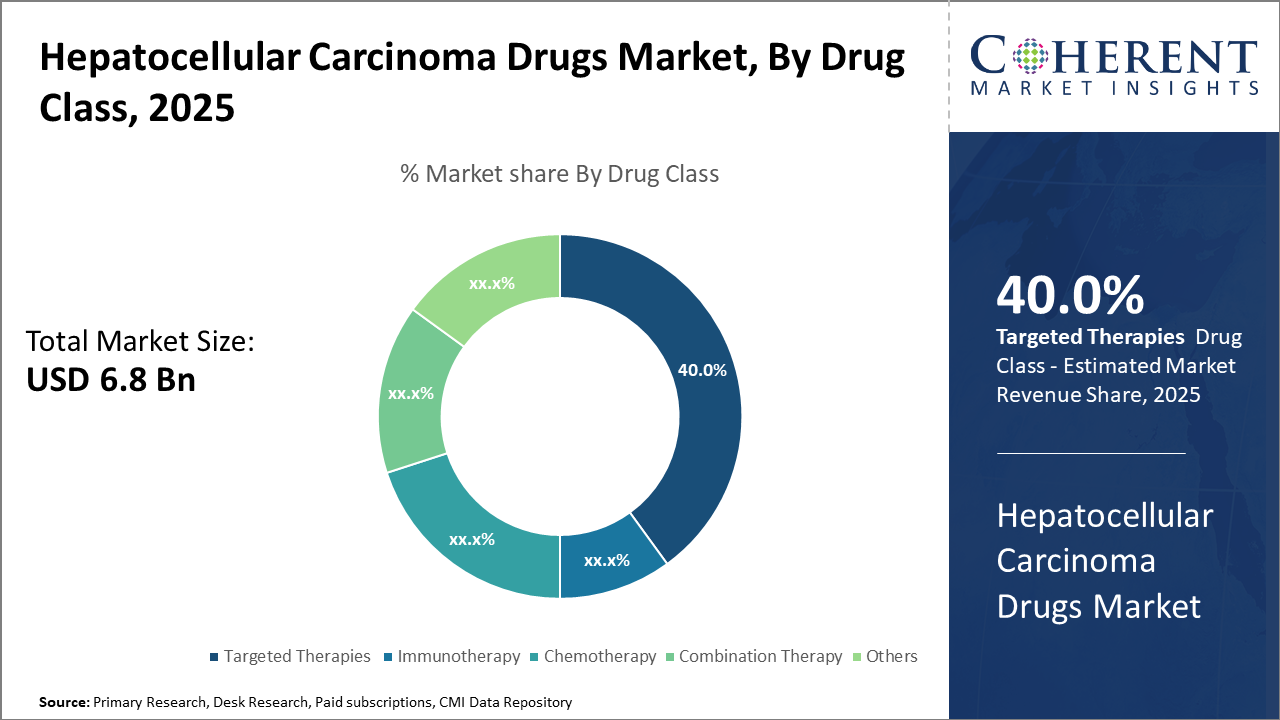
The targeted therapies segment dominates the Hepatocellular Carcinoma Drugs market, driven by recent approvals that improve patient survival significantly.
Immunotherapy is the fastest-growing subsegment, supported by expanding clinical trial data validating combination regimens’ efficacy.
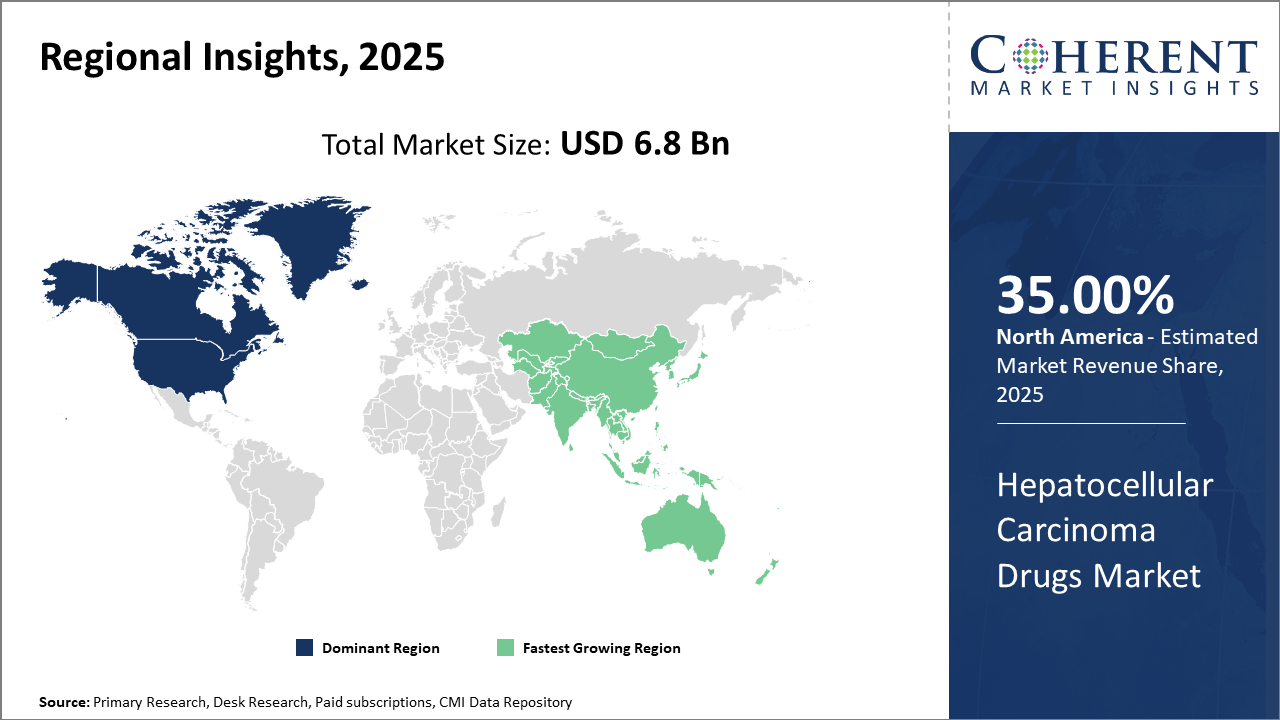
North America holds the largest industry size with approximately 35% of market share, owing to advanced healthcare systems and supportive reimbursement policies.
Asia Pacific is the fastest-growing region due to the increasing prevalence of HCC, growing healthcare investments, and improving drug approval pipelines.
Hepatocellular Carcinoma Drugs Market Segmentation Analysis
To learn more about this report, Download Free Sample
Hepatocellular Carcinoma Drugs Market Insights, By Drug Class
Targeted Therapies dominate the market share with 40%. This dominance results from the proven efficacy of tyrosine kinase inhibitors such as sorafenib and lenvatinib that continue to serve as frontline treatment options globally. Targeted therapies benefit from deep pipeline developments and strategic enhancements like combination protocols with novel agents. Immunotherapy represents the fastest-growing subsegment, experiencing accelerated adoption due to breakthrough immune checkpoint inhibitors and their synergistic combinations, especially post-2023.
Hepatocellular Carcinoma Drugs Market Insights, By Treatment Modality
First-line Treatment is dominating the market share. The dominance stems from the widespread clinical adoption of combination agents such as atezolizumab plus bevacizumab, which accounted for over 45% utilization rates in 2024. These therapies redefine the standard of care in newly diagnosed HCC patients, expanding market revenue across oncology centers. Second-line Treatment holds the fastest growth potential, supported by approvals of drugs like cabozantinib and regorafenib for patients who progress on first-line regimens, increasing options for refractory disease management. Adjuvant Vaccine, though nascent, is gaining relevance through clinical trials targeting early-stage intervention.
Hepatocellular Carcinoma Drugs Market Insights, By Route of Administration
Oral administration dominates due to patient convenience and drug design advancements. Key oral agents such as sorafenib and lenvatinib have established robust market revenue owing to ease of administration and outpatient applicability. Intravenous administration, including monoclonal antibodies and immune checkpoint inhibitors like atezolizumab, is the fastest-growing route driven by expanding immunotherapy use requiring hospital or clinic-based infusion setups. Subcutaneous administration is emerging, offering potential advantages in outpatient settings and patient comfort, but it remains a niche segment.
Hepatocellular Carcinoma Drugs Market Trends
The Hepatocellular Carcinoma Drugs market trend in recent years is characterized by the rapid integration of immuno-oncology agents.
For example, the 2023 FDA approval of immune checkpoint inhibitors combined with vascular endothelial growth factor (VEGF) inhibitors has revolutionized first-line treatment protocols.
This shift is complemented by the rising implementation of liquid biopsy technologies to monitor therapeutic outcomes and detect tumor mutations in real-time, enhancing personalized treatment adaptations.
Additionally, Asia Pacific’s market witnessed a strong push towards biosimilar introductions, responding to affordability challenges and increasing patient access, exemplified by the launch of generic sorafenib products in China and India in 2024.
Hepatocellular Carcinoma Drugs Market Insights, By Geography
To learn more about this report, Download Free Sample
North America Hepatocellular Carcinoma Drugs Market Analysis and Trends
In North America, the Hepatocellular Carcinoma Drugs market dominates, generating nearly 35% of the market share owing to a robust healthcare ecosystem, strong biopharma presence, and advanced reimbursement policies. The U.S. alone contributes over 30% of global oncology drug revenue, supported by government programs such as Medicare facilitating patient access to novel therapies.
Asia Pacific Hepatocellular Carcinoma Drugs Market Analysis and Trends
Meanwhile, Asia Pacific exhibits the fastest growth, with a CAGR exceeding 9% fueled by increasing HCC prevalence linked to endemic hepatitis infections, expanding healthcare infrastructure, and government initiatives promoting cancer screening and treatment awareness. Companies such as Eisai and Bayer have intensified their presence in countries like China and India, launching patient support programs to accelerate adoption.
Hepatocellular Carcinoma Drugs Market Outlook for Key Countries
USA Hepatocellular Carcinoma Drugs Market Analysis and Trends
The U.S. Hepatocellular Carcinoma Drugs market benefits from a favorable regulatory environment and high healthcare expenditure. Notably, the accelerated approval of combination immunotherapies like atezolizumab-bevacizumab strongly impacted treatment protocols in 2023, capturing approximately 40% of market revenue. Major players including Bristol-Myers Squibb and Roche, have invested heavily in clinical research across multiple centers, reinforcing the U.S. as a primary innovation hub. Furthermore, payer strategies encouraging coverage for novel drug regimens contribute to steady business growth.
China Hepatocellular Carcinoma Drugs Market Analysis and Trends
China’s market is rapidly expanding due to a large hepatocellular carcinoma patient base driven by high hepatitis B virus prevalence. Recent government policies aimed at enhancing cancer care infrastructure and reimbursement access have facilitated increased uptake of targeted therapies. Companies such as Eisai and Bayer have launched extensive patient support initiatives, resulting in a surge in market revenue in 2024. Additionally, the introduction of locally manufactured biosimilars has improved affordability, accelerating market penetration in both Tier 1 and Tier 2 cities.
Analyst Opinion
The oncology drug pipeline for hepatocellular carcinoma (HCC) has seen significant capacity expansion in 2024, with over 25 late-stage clinical trials reported worldwide. For instance, the approval of combination therapies such as atezolizumab with bevacizumab has demonstrated superior survival benefits over traditional sorafenib monotherapy, impacting market revenue streams substantially.
Market demand is increasingly driven by expanded patient access programs and healthcare reimbursement reforms, especially in North America and Europe. Real-world data from 2023 indicates an approximate 15% increase in patient uptake due to regulatory emphasis on value-based care models, influencing market share positively in these regions.
Pricing dynamics remain a pivotal micro-indicator; while novel immunotherapies command premium pricing, the introduction of biosimilars and generic small-molecule inhibitors is expected to moderate cost pressures. For example, the launch of generic lenvatinib in 2024 led to a 20% price reduction in select markets, broadening affordability and uptake in emerging economies.
On the demand side, the diversification of treatment protocols to include combination regimens for advanced-stage HCC is expanding therapeutic use cases. Clinical trials showing improved progression-free survival indicate a sustained growth trajectory potentially boosting market revenue from multi-drug treatments by over 12% annually.
Market Scope
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2025 | Market Size in 2025: | USD 6.8 billion |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 8.1% | 2032 Value Projection: | USD 11.5 billion |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: | Bayer AG, Bristol-Myers Squibb, Eisai Co., Ltd., F. Hoffmann-La Roche AG, Novartis AG, Pfizer Inc., Merck & Co., Inc., AstraZeneca PLC, Sanofi S.A., Johnson & Johnson, Taiho Pharmaceutical Co., Ltd., Exelixis, Inc., Ipsen S.A. | ||
| Growth Drivers: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Hepatocellular Carcinoma Drugs Market Growth Factors
Key growth drivers include the rising incidence of liver cirrhosis and viral hepatitis as primary risk factors fueling hepatocellular carcinoma cases globally. Industry trends reveal that hepatitis B and C infections account for approximately 80% of HCC cases, emphasizing the growing patient pool. Secondly, advancements in molecular diagnostics and precision medicine have accelerated targeted drug development, significantly improving treatment efficacy and influencing market growth. Thirdly, favorable regulatory frameworks enabling faster approvals for breakthrough therapies have expanded market access, especially in the U.S. and Europe. Lastly, increasing healthcare expenditure and government initiatives to bolster cancer care infrastructure in emerging economies like China and India are catalyzing market revenue growth, creating unprecedented business growth opportunities.
Hepatocellular Carcinoma Drugs Market Development
In March 2025, Junshi Biosciences received approval from China's NMPA for a combination therapy of TUOYI® (toripalimab) and bevacizumab for the first-line treatment of unresectable or metastatic hepatocellular carcinoma (HCC). This approval marks a significant development for advanced liver cancer treatment in China, as it provides a new option for patients in the early stages of their treatment.
In April 2025, the US Food and Drug Administration (FDA) approved the immunotherapy combination of nivolumab (Opdivo) and ipilimumab (Yervoy) in April 2025 for the first-line treatment of adults with unresectable or metastatic hepatocellular carcinoma (HCC).
Key Players
Leading Companies of the Market
Bayer AG
Bristol-Myers Squibb
Eisai Co., Ltd.
F. Hoffmann-La Roche AG
Novartis AG
Pfizer Inc.
Merck & Co., Inc.
AstraZeneca PLC
Sanofi S.A.
Johnson & Johnson
Taiho Pharmaceutical Co., Ltd.
Exelixis, Inc.
Ipsen S.A.
Competitive strategies among market companies include aggressive in-licensing and co-development initiatives. For example, Bristol-Myers Squibb and Exelixis partnered on immuno-oncology combinations leading to successful Phase III results and global approvals, boosting their market share by over 10% in 2024. Similarly, Bayer has utilized strategic acquisitions to enhance its targeted therapy portfolio, generating sustained revenue growth in Asia Pacific markets.
Hepatocellular Carcinoma Drugs Market Future Outlook
The future of HCC drug development lies in precision medicine, where genetic profiling guides therapeutic decisions. Combination therapies that unite targeted agents, immunotherapies, and localized treatments are expected to define next-generation protocols. Drug developers are exploring new molecular targets and delivery systems to enhance efficacy while minimizing hepatotoxicity. Advancements in diagnostic imaging and biomarker testing will further refine treatment selection and monitoring, enabling more individualized and adaptive therapeutic strategies for liver cancer management.
Hepatocellular Carcinoma Drugs Market Historical Analysis
Hepatocellular carcinoma treatment initially relied on surgical interventions and locoregional therapies such as ablation and embolization. The introduction of the first systemic therapy for HCC was a major turning point, as it offered a pharmacological pathway for patients with advanced-stage disease. Over time, the development of additional targeted drugs and immune checkpoint inhibitors broadened the therapeutic landscape, allowing combination regimens to become standard in clinical practice. Research into liver-specific metabolism and drug resistance mechanisms has also shaped the evolution of more selective and tolerable treatment options.
Sources
Primary Research interviews:
Oncology Pharmacologists
Hepatobiliary Surgeons
Clinical Oncologists
Drug Development Scientists
Databases:
PubMed / NCBI
EMA / FDA Drug Approval Databases
GlobalData HCC Pipeline Reports
Magazines:
Cancer Therapy Advisor
PharmaTimes
Targeted Oncology
Clinical Oncology News
Journals:
Journal of Hepatology
Hepatology
Cancer Medicine
Annals of Oncology
Newspapers:
The Guardian (Health)
The New York Times (Science & Health)
The Economic Times (Pharma)
Financial Times (Healthcare)
Associations:
American Society of Clinical Oncology (ASCO)
European Society for Medical Oncology (ESMO)
AASLD
National Comprehensive Cancer Network (NCCN)
Share
Share
About Author
Ghanshyam Shrivastava - With over 20 years of experience in the management consulting and research, Ghanshyam Shrivastava serves as a Principal Consultant, bringing extensive expertise in biologics and biosimilars. His primary expertise lies in areas such as market entry and expansion strategy, competitive intelligence, and strategic transformation across diversified portfolio of various drugs used for different therapeutic category and APIs. He excels at identifying key challenges faced by clients and providing robust solutions to enhance their strategic decision-making capabilities. His comprehensive understanding of the market ensures valuable contributions to research reports and business decisions.
Ghanshyam is a sought-after speaker at industry conferences and contributes to various publications on pharma industry.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients