Hemostasis Valve Market Size and Forecast – 2026 – 2033
The Global Hemostasis Valve Market size is estimated to be valued at USD 280 million in 2026 and is expected to reach USD 420 million by 2033, exhibiting a compound annual growth rate (CAGR) of 6.2% from 2026 to 2033.
Global Hemostasis Valve Market Overview
Hemostasis valves are medical devices designed to control blood loss during catheter-based interventional procedures. These valves provide a sealed access point that allows medical instruments to pass through while minimizing blood leakage. Hemostasis valves are commonly used in cardiovascular, neurovascular, and interventional radiology procedures. The products emphasize leak prevention, ease of operation, and compatibility with various catheter sizes to support procedural efficiency and patient safety.
Key Takeaways
The cardiovascular application segment dominates the market, accounting for over 45% of the market share due to increasing minimally invasive procedures worldwide.
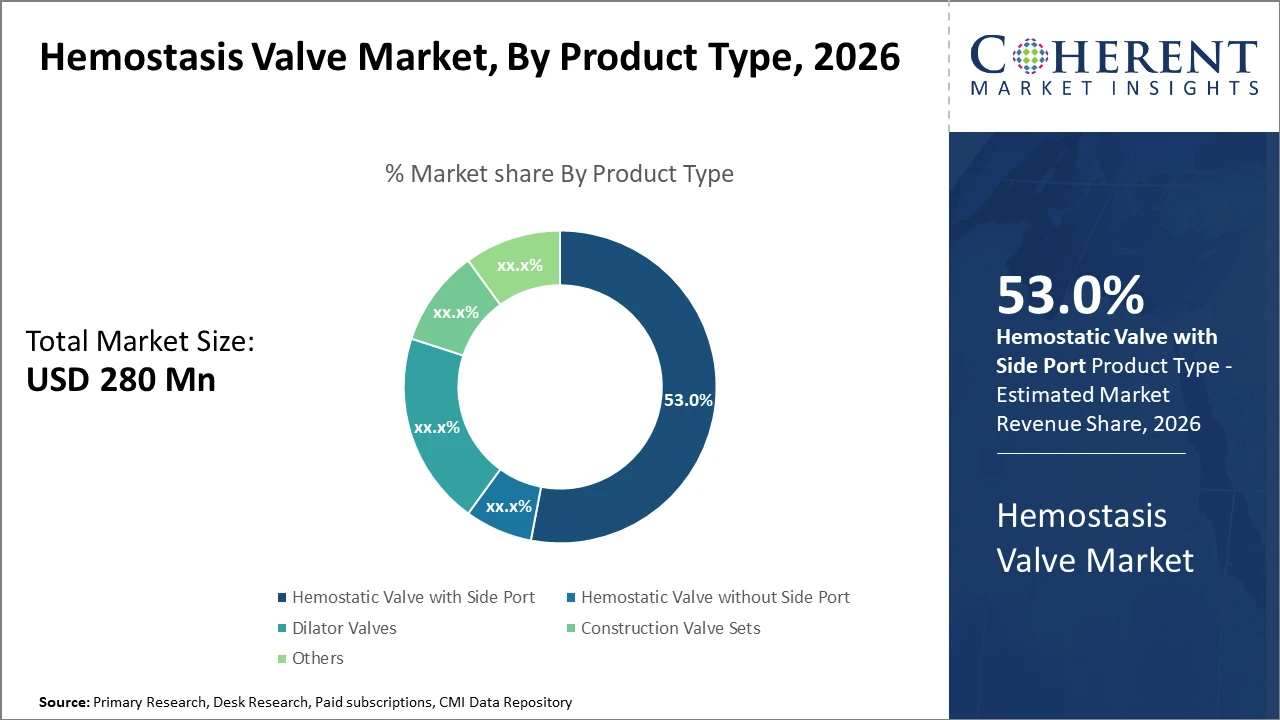
The Hemostatic Valve with Side Port product type leads with a commanding 53% share, reflecting widespread clinical preference for enhanced device functionality.
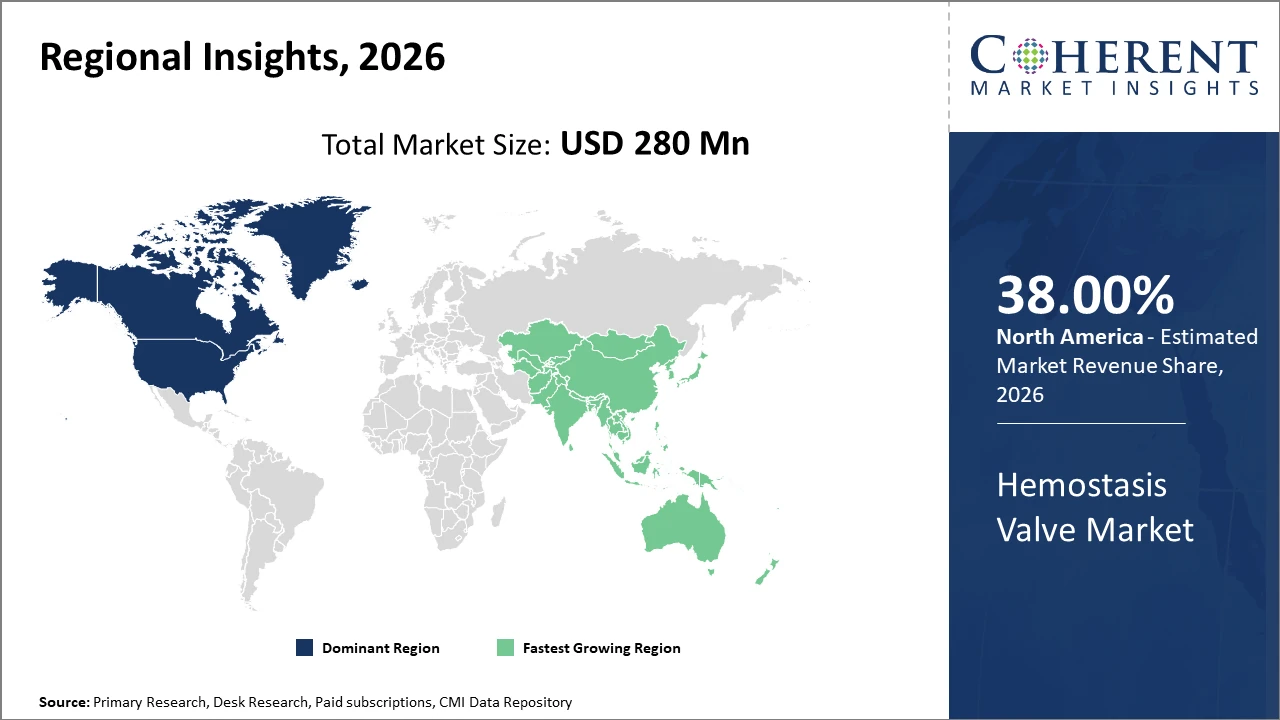
Regionally, North America maintains leadership with approximately 38% market share, driven by a robust healthcare ecosystem and technological adoption, while Asia Pacific emerges as the fastest-growing region, registering a CAGR exceeding 7%, fueled by rising surgical interventions and expanding healthcare infrastructure.
Hemostasis Valve Market Segmentation Analysis
To learn more about this report, Download Free Sample
Hemostasis Valve Market Insights, By Product Type
Hemostatic Valve with Side Port dominates the market share, accounting for 53%, due to their multifunctionality, allowing the introduction of ancillary devices during procedures without blood loss. This versatility enhances procedural efficiency, making it the preferred choice in cardiovascular and urology interventions. The fastest growing subsegment is Dilator Valves, which is gaining traction owing to their ease of insertion and compatibility with varied catheter sizes. Advancements in dilator valve materials have minimized resistance during catheter navigation, fueling adoption in interventional radiology and gastrointestinal procedures.
Hemostasis Valve Market Insights, By Application
Cardiovascular applications hold the lead market share, driven by an increase in minimally invasive cardiac catheterizations and electrophysiology procedures, supported by technologies reducing procedural complications. Urology is identified as the fastest-growing segment with increased adoption of endourological surgeries using hemostasis valves for blood loss prevention. Rising incidences of urological disorders and investment in ambulatory surgical centers advance demand in this segment. Gastroenterology continues to grow steadily as more endoscopic therapeutic procedures necessitate effective hemostasis. Interventional Radiology benefits from the trend toward non-surgical interventions, with valves facilitating safe catheter exchanges.
Hemostasis Valve Market Insights, By End-User
Hospitals dominate market share, given their extensive surgical volumes, comprehensive procedural scope, and strong procurement capabilities to adopt advanced hemostasis valves. Ambulatory Surgical Centers represent the fastest-growing end-user segment as outpatient procedures increase worldwide. Their emphasis on quick turnover times and cost containment aligns with demand for efficient valve systems designed for minimally invasive interventions. Specialty Clinics and Diagnostic Centers maintain a niche but growing relevance, particularly as diagnostic interventional procedures rise.
Hemostasis Valve Market Trends
The market is increasingly influenced by three pivotal trends.
First, digital integration through smart valves provides real-time intraoperative monitoring, improving surgical precision and patient safety—a factor that led to a 20% adoption increase in leading North American hospitals in 2025.
Second, growing preference for single-use, disposable valves aims to mitigate infection risks, catalyzing a 30% surge in demand in the US and Europe over 2023-2026.
Lastly, robotics-assisted surgical procedures have accelerated valve adoption in Europe and Asia Pacific, with robotic interventions rising by 15%, linking directly to enhanced valve functionalities tailored for these complex surgeries.
Hemostasis Valve Market Insights, By Geography
To learn more about this report, Download Free Sample
North America Hemostasis Valve Market Analysis and Trends
In North America, the Hemostasis Valve Market dominates with an approximate 38% industry share. This leadership stems from the region’s advanced healthcare infrastructure, presence of major market companies, and significant volume of cardiovascular and urology interventions. Government initiatives encouraging minimally invasive surgeries and reimbursement policies further catalyze market revenue.
Asia Pacific Hemostasis Valve Market Analysis and Trends
Meanwhile, Asia Pacific exhibits the fastest growth with an estimated CAGR of over 7%. Rapid expansion of healthcare infrastructure in China and India, coupled with increasing procedural awareness and affordability, has boosted demand. Notably, manufacturing hubs in the region facilitate supply chain efficiency, contributing to market dynamics.
Hemostasis Valve Market Outlook for Key Countries
USA Hemostasis Valve Market Analysis and Trends
The USA’s market is the largest contributor to regional dominance, driven by a high volume of minimally invasive procedures and advanced healthcare delivery systems. In 2025, cardiovascular surgeries utilizing hemostasis valves increased by over 12%, backed by FDA-cleared innovations and robust hospital adoption. Leading companies such as Medtronic and Boston Scientific have extensive portfolios supported by clinical trials validated in the US market, enhancing market share and fueling ongoing business growth strategies.
China Hemostasis Valve Market Analysis and Trends
China exhibits remarkable market revenue growth, supported by rising investments in healthcare infrastructure and government initiatives promoting advanced surgical technologies. The growing prevalence of chronic diseases and increased procedural volumes in tertiary hospitals have stimulated demand, with over 20% annual growth reported in the hemostasis valve segment in 2025. Locally based companies also benefit from streamlined regulatory approvals, accelerating market penetration.
Analyst Opinion
One significant actionable insight in the market involves the increasing demand in the cardiovascular segment, reflected by a 12% increase in minimally invasive cardiac surgeries in 2025, which directly boosts valve utilization. Regulations mandating blood conservation practices in Europe also contributed to an 8% rise in regional imports, reinforcing supply-side growth.
Pricing strategies of hemostasis valves have shown upward trends due to material innovations such as heparin-coated valves reducing thrombogenic risks. For instance, 2026 data from leading hospitals indicate a 5% premium pricing acceptance for valves with advanced coatings, stimulating market revenue growth.
Use case diversification is accelerating, with urology and peripheral interventions seeing a 15% rise in procedural adoption of hemostasis valves in North America during 2025, driving demand-side metrics. This broadening clinical scope further pressures players to expand product portfolios, influencing market share dynamics.
On a nano-indicator level, manufacturing capacity expansions were notable with select production facilities increasing output by 20% in 2023 to meet rising demand in Asia Pacific. Export data showed a 10% surge from key manufacturing hubs, underlining robust market growth trajectories.
Market Scope
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2025 | Market Size in 2026: | USD 280 million |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2026 To 2033 |
| Forecast Period 2026 to 2033 CAGR: | 6.2% | 2033 Value Projection: | USD 420 million |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: | Vygon SA, Applied Medical Resources Corporation, LeMaitre Vascular, Inc., Stryker Corporation, Cardinal Health, Inc., AngioDynamics, Inc., Baxter International Inc., RADI Medical Systems, Haemonetics Corporation, MicroVention, Inc. | ||
| Growth Drivers: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Hemostasis Valve Market Growth Factors
The Hemostasis Valve Market’s growth is fueled primarily by the rising prevalence of cardiovascular diseases worldwide, which escalated procedural volumes by over 10% in 2025 alone. Increasing healthcare infrastructure investments, especially in emerging economies, enable broader access to minimally invasive surgical tools, driving market revenue. Innovations in valve materials and designs, such as biocompatible coatings, prolong device usability and reduce complications, enhancing physician preference and client acceptance rates. Additionally, stringent regulatory frameworks targeting surgical safety and blood management protocols have positioned hemostasis valves as critical devices, thus expanding market scope and underpinning robust market growth.
Hemostasis Valve Market Development
In 2019, Merit Medical Systems launched the PhD (Push, Hold, and Deliver) polycarbonate hemostasis valve, featuring an advanced dual-seal design that enables true one-handed operation. The valve was engineered to minimize blood loss during interventional procedures while supporting high-pressure injections, improving procedural efficiency and clinician control in catheter-based interventions.
Key Players
Leading Companies of the Market
Vygon SA
Applied Medical Resources Corporation
LeMaitre Vascular, Inc.
Stryker Corporation
Cardinal Health, Inc.
AngioDynamics, Inc.
Baxter International Inc.
RADI Medical Systems
Haemonetics Corporation
MicroVention, Inc.
Several market companies have pursued strategic acquisitions and R&D collaborations to boost innovation pipelines and global reach. For example, Medtronic’s recent acquisition of a specialty hemostasis valve manufacturer enabled portfolio diversification, contributing to a 9% revenue increase in 2025. Boston Scientific’s partnership with leading vascular centers has enhanced clinical efficacy trials for valves, generating market confidence reflected in heightened adoption rates.
Hemostasis Valve Market Future Outlook
Future growth in the hemostasis valve market will be driven by the continued expansion of minimally invasive procedures, especially in cardiovascular and neurological interventions. Innovation will likely emphasize multi-port access systems, lower profile designs, and improved biocompatible materials that reduce complications and enhance user handling. Integration with imaging guidance and robotics may further streamline interventional workflows. As the volume of outpatient and hybrid operating room procedures increases, hemostasis valves that support flexible, rapid access with secure sealing will be in high demand. Overall, the market will benefit from broader adoption of structural heart and peripheral vascular interventions.
Hemostasis Valve Market Historical Analysis
The hemostasis valve market has evolved as minimally invasive interventional procedures expanded across cardiovascular, neurovascular, and peripheral vascular medicine. Hemostasis valves were developed to address the critical need to maintain a sealed access point during catheterization and device exchange, preventing blood loss and reducing procedure risk. Early valves were basic mechanical devices with limited compatibility and reliability; however, improvements in materials, sealing mechanisms, and ergonomic design proliferated as interventional volumes increased. The rise of catheter-based therapies—for angioplasty, stenting, and electrophysiology—accelerated demand for hemostasis solutions that are leak-proof, easy to operate, and compatible with a wide range of catheter sizes and guidewires.
Sources
Primary Research Interviews:
Interventional Cardiologists
Cath Lab Technicians
Medical Device Engineers
Procurement Managers
Databases:
WHO Device Reports
OECD Health Equipment Data
Magazines:
Cath Lab Digest
Medical Device Network
Endovascular Today
Cardiovascular Business
MedTech Review
Journals:
Journal of Interventional Cardiology
Catheterization and Cardiovascular Interventions
Medical Devices Journal, Vascular Medicine Journal
Clinical Engineering
Newspapers:
Financial Times (MedTech)
Reuters Health
Bloomberg Healthcare
The Hindu BusinessLine
Economic Times
Associations:
Society for Cardiovascular Angiography & Interventions
Medical Device Manufacturers Association
American College of Cardiology
European Society of Cardiology
AdvaMed
? Frequently Asked Questions
1. Who are the dominant players in the Hemostasis Valve Market?
Leading companies include Medtronic plc, Becton Dickinson and Company, Boston Scientific Corporation, Terumo Corporation, and Cook Medical, among others, which drive innovation and market growth.
2. What will be the size of the Hemostasis Valve Market in the coming years?
The market size is projected to increase from USD 280 million in 2026 to USD 420 million by 2033 with a CAGR of 6.2%, driven by rising procedural volume and technological advancements.
3. Which end-user industry has the largest growth opportunity?
Hospitals and ambulatory surgical centers represent the largest end-users due to increased minimally invasive surgeries and greater focus on patient safety protocols.
4. How will market development trends evolve over the next five years?
The Hemostasis Valve Market trends will shift towards digitization, robotic integration, and eco-friendly device options, enhancing precision and safety during surgical interventions.
5. What is the nature of the competitive landscape and challenges in the Hemostasis Valve Market?
The market is competitive, with strategic acquisitions, R&D collaborations, and innovation as primary business growth strategies. Challenges include regulatory compliance and pricing pressures.
6. What go-to-market strategies are commonly adopted in the Hemostasis Valve Market?
Companies focus on strategic partnerships with healthcare providers, portfolio diversification, and clinical trial validations to enhance adoption and market penetration.
Share
Share
About Author
Komal Dighe is a Management Consultant with over 8 years of experience in market research and consulting. She excels in managing and delivering high-quality insights and solutions in Health-tech Consulting reports. Her expertise encompasses conducting both primary and secondary research, effectively addressing client requirements, and excelling in market estimation and forecast. Her comprehensive approach ensures that clients receive thorough and accurate analyses, enabling them to make informed decisions and capitalize on market opportunities.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients