Hemorrhagic Shock Treatment Market is estimated to be valued at USD 224.6 Mn in 2025 and is expected to reach USD 303.6 Mn in 2032, exhibiting a compound annual growth rate (CAGR) of 4.4% from 2025 to 2032.
Analysts’ views on Global Hemorrhagic Shock Treatment Market:
Treatment of trauma patients with hemorrhagic shock is complex and difficult. Despite the knowledge gained in recent decades about the pathophysiology of hemorrhagic shock in trauma patients, the mortality rate of these patients is high. In the acute phase of bleeding, the therapeutic priority is to stop the bleeding as quickly as possible. While this bleeding is uncontrolled, the physician must maintain the oxygen supply to limit tissue hypoxia, inflammation, and organ dysfunction. This process includes fluid resuscitation, the use of vasopressors, and blood transfusion to prevent or correct acute coagulopathy from trauma. The optimal resuscitation strategy is controversial. Optimal therapies with clear goals for fluid resuscitation, blood pressure, and hemoglobin levels must be established to guide resuscitation and limit the risk of fluid overload and transfusion.
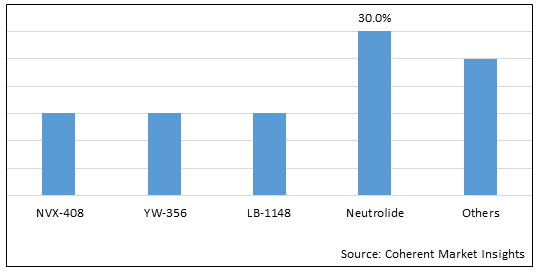
Figure 1. Global Hemorrhagic Shock Treatment Market Share (%), By Drug Type, 2025
To learn more about this report, Download Free Sample
Global Hemorrhagic Shock Treatment Market– Drivers
Increasing research and development activities in hemorrhagic shock treatment
Increasing research and development in the field of hemorrhagic shock treatment is expected to drive the global hemorrhagic shock treatment market growth over the forecast period. For instance, on March 25, 2023, John Wiley & Sons, Inc, U.S. based multinational publishing company published an article explaining several patient anti-shock therapies mitigate the damage caused by ischemia and reperfusion, enabling the subjects to better tolerate acute blood loss and hemorrhagic shock. Ethinyl estradiol sulfate, an estrogen derivative, reduces damage caused by apoptosis, nitric oxide (NO) production, and inflammation by improving cardiovascular performance in rodent and pig models, improving O2 delivery, and contributing to the restoration of the pre-shock state.
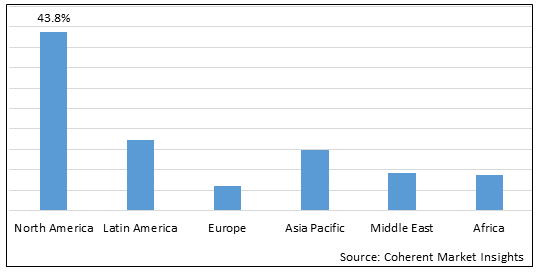
Figure 2. Global Hemorrhagic Shock Treatment Market Value (US$ Million), By Region, 2025
To learn more about this report, Download Free Sample
Global Hemorrhagic Shock Treatment Market - Regional Analysis
Among region, North America is estimated to hold a dominant position in the global hemorrhagic shock treatment market over the forecast period, owing to increasing research and development in treatment for hemorrhagic shock. For instance, on March 25, 2025, John Wiley & Sons, Inc published an article explaining polyethylene glycol 20,000 is a solution that can be used in "minimum resuscitation", which can advantageously reduce the logistical burden in severe combat conditions. The mechanism of PEG-20 K targets the microvasculature and the unique solute can exit the vasculature into the interstitial space, but remains cell impermeable and too large to pass through the capillaries, providing an oncotic force to prevent cell swelling and maintain microcirculation. In a study comparing small volumes of fluids given in hemorrhagic shock, a preclinical study in all pigs given PEG-20 K survived compared to less than 20% of pigs given other fluids (whole blood and hetastarch). PEG-20 K restored mean arterial pressure, intravascular volume, and capillary perfusion.
Global Hemorrhagic Shock Treatment Market – Impact of Coronavirus (COVID-19) Pandemic
Since the COVID-19 virus outbreak in December 2019, the disease has spread to over 100 countries across the globe, and the World Health Organization declared it a public health emergency on January 30, 2020.
COVID-19 affected the economy in three main ways: by directly affecting the production and demand of drugs, by creating disruptions in distribution channels, and through its financial impact on firms and financial markets. Due to nationwide lockdowns, several countries, such as China, India, Saudi Arabia, the U.A.E., Egypt, and others faced problems regarding the transportation of drugs from one place to another.
However, the COVID-19 pandemic has negatively impacted the global hemorrhagic shock treatment market. For instance, on February 15, 2022, Gavin Publishers, published an article explaining the clinical manifestations of this pathology range from asymptomatic patients, headache, fever, dyspnoea, cough, anosmia/ageusia, myalgia, respiratory failure and multiorgan failure. However, it is important to highlight the disability that this disease can cause, causing important complications after recovery, thus encompassing an endless number of symptoms that we call post-COVID-19 syndrome. Post-COVID- 19 syndrome is increasingly recognized as a clinical entity characterized by symptoms that persist for more than 3 weeks after the diagnosis of COVID-19. Its incidence varies from 10 to 35% of cases, yet it has been shown that up to 85% of hospitalized patients present with post-COVID-19 syndrome.19 However, there is currently no worldwide consensus on the definition and classification of post-COVID-19 syndrome.
Hemorrhagic Shock Treatment Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 224.6 Mn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 4.4% | 2032 Value Projection: | USD 303.6 Mn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Biomedica Management Corporation, NuvOxPharma LLC, Leading BioSciences, Inc, F. Hoffmann-La Roche Ltd, Bristol-Myers Squibb Company, Boehringer Ingelheim International GmbH, Johnson & Johnson Services Inc, AstraZeneca, Daiichi Sankyo Company, Limited, Teva Pharmaceutical Industries Ltd., Novartis AG, ZydusCadila, Amneal Pharmaceuticals LLC. |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Global Hemorrhagic Shock Treatment Market Segmentation:
Global hemorrhagic shock treatment market report is segmented into drug type, distribution channel, and region.
Based on drug type, the global hemorrhagic shock treatment market is segmented into NVX-408, YW-356, LB-1148, neutrolide, and others. Out of which, neutrolide segment is expected to dominate the market due to the increasing launch of products.
Based on distribution channels, the hospital pharmacies is segmented into hospital pharmacies, retail pharmacies, and online channels. Among these, the hospital pharmacies segment is expected to dominate the market over the forecast period, owing to increasing treatments and medicines.
Based on Region, the global hemorrhagic shock treatment market is segmented into North America, Latin America, Europe, Asia Pacific, Middle East & Africa. Among these, North America is expected to dominate the market over the forecast period due to increased research and development activities.
Among all the segmentation, drug type segment is expected to dominate the global hemorrhagic shock treatment market. For instance, on March 25, 2023, John Wiley & Sons, Inc published an article explaining inhibition of the complement pathway has also been studied as an anti-shock target, since activation of the complement system is associated with the physiological response to trauma and hemorrhage, which cause tissue damage. Recombinant human decay-accelerating factor (DAF), an inhibitor. the effects of the complement system and C1-esterase inhibitor (C1INH) have been studied in animal models.
Global Hemorrhagic Shock Treatment Market Cross Sectional Analysis:
Introduction of treatments of hemorrhagic shock treatment in North America region is expected to drive the growth of drug type segment in the region. For instance, in September 2022 Merck & Co., Inc, pharmaceutical company based in U.S. published article explaining that surgical control of bleeding is the primary goal in bleeding disorders. Volume replacement is associated with and does not precede surgical control. Blood products and crystalloid solutions are used for resuscitation; however, patients who are likely to require massive transfusions receive red blood cells, fresh frozen plasma, and platelets earlier and in a 1:1:1 ratio. Failure is usually a sign of insufficient volume or undetected ongoing bleeding. Vasopressors may be tried in refractory hemorrhagic shock, but only after adequate blood volume has been restored and bleeding has been controlled; administration of vasopressors prior to this may worsen the results.
Global Hemorrhagic Shock Treatment Market: Key Developments
For instance, on March 20, 2023, Pharmazz, Inc., a biopharmaceutical company focused on developing and commercializing novel therapeutics to treat patients in critical care, announced two key publications. These publications add to the understanding of adrenergic receptors in the treatment of shock.Controls of central and peripheral blood pressure are critical in treating patients with hemorrhagic/hypovolemic shock. It has been demonstrated that alpha2-adrenergic receptors could be a suitable target for managing hypovolemic shock.
On May 14, 2020, Pharmazz, Inc., a biopharmaceutical company focused on the development and commercialization of novel therapeutics to treat patients in critical care, announced that it has received marketing authorization for centhaquine, a first-in-class drug, to manage patients with hypovolemic shock from the Indian regulatory agency. Centhaquine is likely to be a transformational therapy for hypovolemic shock because it ameliorates key drivers of mortality.
Global Hemorrhagic Shock Treatment Market: Key Trends
Increasing research and the introduction of newly launched drugs can drive the growth of the global hemorrhagic shock treatment market. For instance, on September 13, 2022, Pharmazz, Inc, a biopharmaceutical company focused on developing and commercializing novel therapeutics to treat patients in critical care, will present a poster on its positive Phase III study of centhaquine as a resuscitative agent in hypovolemic shock patients at the Military Health System Research Symposium (MHSRS) September 12-15, 2022. Centhaquine is the company’s resuscitative agent free of arterial constriction and is commercially available to healthcare professionals in India with the brand name, Lyfaquin.
Global Hemorrhagic Shock Treatment Market: Restraints
Risk related to hemorrhagic shock treatment
The risk of developing adverse reactions on several factors related to treatment can hamper the growth of the global hemorrhagic shock treatment market. For instance, on July 27, 2020, Daiichi Sankyo Company, Limited, a company focused on pharmaceutical therapies explained the safety of ENHERTU was evaluated in a pooled analysis of patients with unresectable or metastatic HER2-positive breast cancer who received at least one dose of ENHERTU 5.4 mg/kg in DESTINY-Breast01 and study DS8201-A-J101. ENHERTU was administered by intravenous infusion once every three weeks. The median duration of treatment was 7 months. Serious adverse reactions occurred in 20% of patients receiving ENHERTU. Serious adverse reactions in >1% of patients who received ENHERTU were interstitial lung disease, pneumonia, vomiting, nausea, cellulitis, hypokalemia, and intestinal obstruction. Fatalities due to adverse reactions occurred in 4.3% of patients including interstitial lung disease, and the following events occurred in one patient each: acute hepatic failure/acute kidney injury, general physical health deterioration, pneumonia, and hemorrhagic shock.
To counterbalance this restrain, more effective treatments should be introduced to reduce the adverse reactions of drugs.
Global Hemorrhagic Shock Treatment Market - Key Players
Major players operating in the global hemorrhagic shock treatment market include Biomedica Management Corporation, NuvOxPharma LLC, Leading BioSciences, Inc, F. Hoffmann-La Roche Ltd, Bristol-Myers Squibb Company, Boehringer Ingelheim International GmbH, Johnson & Johnson Services Inc, AstraZeneca, Daiichi Sankyo Company, Limited, Teva Pharmaceutical Industries Ltd., Novartis AG, ZydusCadila, Amneal Pharmaceuticals LLC.
*Definition: Hemorrhagic shock is shock caused by massive blood loss, which can be caused by internal or external bleeding. Hemorrhagic shock can be life-threatening and should be treated as a medical emergency. There are several types of medical shocks. Hypovolemic shock occurs when the body begins to shut down due to a large loss of blood or fluid. When hypovolemic shock is caused by blood loss, it is called hemorrhagic shock. People with injuries that cause heavy bleeding may develop bleeding disorders if the bleeding does not stop immediately.
Share
Share
About Author
Ghanshyam Shrivastava - With over 20 years of experience in the management consulting and research, Ghanshyam Shrivastava serves as a Principal Consultant, bringing extensive expertise in biologics and biosimilars. His primary expertise lies in areas such as market entry and expansion strategy, competitive intelligence, and strategic transformation across diversified portfolio of various drugs used for different therapeutic category and APIs. He excels at identifying key challenges faced by clients and providing robust solutions to enhance their strategic decision-making capabilities. His comprehensive understanding of the market ensures valuable contributions to research reports and business decisions.
Ghanshyam is a sought-after speaker at industry conferences and contributes to various publications on pharma industry.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients