Hemoglobinopathy Market is estimated to be valued at USD 714.4 Mn in 2025 and is expected to reach USD 1,067.1 Mn in 2032, exhibiting a compound annual growth rate (CAGR) of 5.9% from 2025 to 2032.
Analysts’ Views on Global Hemoglobinopathy Market:
Increasing government initiatives is expected to drive the global hemoglobinopathy market over the forecast period. For instance, in August 2020, a Thalassemia Screening and Counselling Centre was launched at Indian Red Cross Society’s National Headquarters Blood Bank, to administer adequate therapy to those affected enabling them lead a better life and preventing the birth of children affected with hemoglobinopathies, through carrier screening, genetic counselling and prenatal diagnosis.
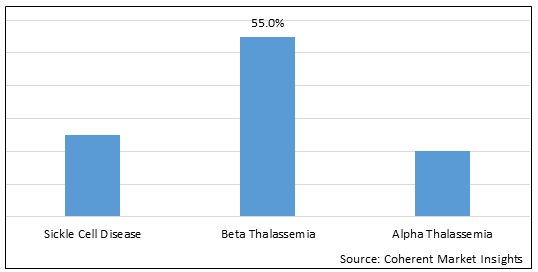
Figure 1. Global Hemoglobinopathy Market Share (%), by Indication, 2025
To learn more about this report, Download Free Sample
Global Hemoglobinopathy Market – Driver
Increasing awareness programs for sickle cell disease
Increasing awareness programs by government bodies is expected to drive the hemoglobinopathy market over the forecast period. For instance, in November 2020, The European Hematology Association (EHA) Topics-in-Focus Hemoglobinopathies Program (focus on Sickle Cell Disease) was launched to expand awareness and education about these increasingly common genetic diseases in Europe, among healthcare professionals, patients and the general population.
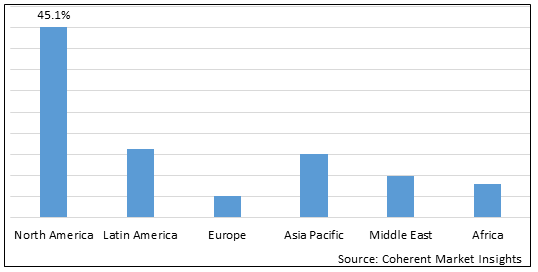
Figure 2. Global Hemoglobinopathy Market Value (US$ Million), by Region, 2025
To learn more about this report, Download Free Sample
Global Hemoglobinopathy Market- Regional Analysis
Among regions, North America is estimated to hold a dominant position in the global hemoglobinopathy market over the forecast period. North America holds 45.1% of the market share due to the increasing prevalence of Beta Thalassemia in the region. For instance, in December 2020, according to a report published on National Center for Biotechnology Information (NCBI), stated that in the U.S., the prevalence of beta thalassemia has increased approximately 7.5% over the last 50 years.
Global Hemoglobinopathy Market – Impact of Coronavirus (COVID-19) Pandemic
Since the COVID-19 virus outbreak in December 2019, the disease spread to over 100 countries across the globe, and the World Health Organization declared it a public health emergency on January 30, 2020.
COVID-19 affected the economy in three main ways: by directly affecting the production and demand of drugs, by creating disruptions in distribution channels, and through its financial impact on firms and financial markets. Due to nationwide lockdowns, several countries such as China, India, Saudi Arabia, the U.A.E., Egypt, and others are facing problems with the transportation of drugs from one place to another.
The COVID-19 pandemic had a negative impact on the global hemoglobinopathy market. For instance, in November 2022, according to a report published by National Center for Biotechnology Information (NCBI), stated that there was a shortage of bone marrow donations globally, due to COVID-19.
Hemoglobinopathy Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 714.4 Mn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 5.9% | 2032 Value Projection: | USD 1,067.1 Mn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Anamol Laboratories Pvt. Ltd., Bio-Rad Laboratories, Inc., PerkinElmer Inc., Zentech, Sebia, Sysmex Europe SE, Nanjing Poclight Biotechnology Co., Ltd, Abbott, Danaher, ReachBio LLC, Shenzhen Mindray Bio-Medical Electronics Co., Ltd., Nexcelom Bioscience LLC., NIHON KOHDEN CORPORATION, Siemens Healthcare GmbH, CRISPR Therapeutics. |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Global Hemoglobinopathy Market Segmentation:
The global hemoglobinopathy market report is segmented into Test Type, Indication, End User and Region
Based on Test Type, the market is segmented into Routine Red Blood Cell (RBC) count, Genetic Testing, Hemoglobin by high performance liquid chromatography, Hemoglobin isoelectric focusing ( Hb IEF), Hemoglobin electrophoresis (Hb ELP), Hemoglobin solubility test. Out of which, the Routine Red Blood Cell (RBC) count segment is expected to dominate the hemoglobinopathy market during the forecast period and this is due to the increase in the usage of Routine Red Blood Cell (RBC) count for the hemoglobinopathy testing.
Based on Indication, the hemoglobinopathy market is segmented into sickle cell disease, beta thalassemia, alpha thalassemia. Beta Thalassemia segment is expected to dominate the market over the forecast period and this is attributed to the increasing prevalence of sickle cell disease.
Based on End User, the market is segmented into hospitals, diagnostic laboratories, clinics. Out of which, the hospital segment is expected to dominate the market over the forecast period and this is attributed to the increase in the number of new hospitals for the management of sickle cell disease over the forecast period.
Among all segmentation, indication segment has the highest potential due to the increasing prevalence of beta-thalassemia. For instance, in June 2021, according to the data published by bluebird bio, Inc., a biotechnology company, stated that about 1.5% of the global population (80-90 million people) are carriers of beta-thalassemia.
Global Hemoglobinopathy Market Cross Sectional Analysis:
In indication segment, beta-thalassemia hold a dominant segment in North America region due to the increasing prevalence of beta-thalassemia. For instance, on April 4 2023, according to Centers for Disease Control and Prevention (CDC), stated that beta-thalassemia, affects at least 1000 people in the U.S.
Global Hemoglobinopathy Market: Key Developments
Global Hemoglobinopathy Market: Key Trends
Increasing government initiatives for the management and treatment of hemoglobinopathy
Increasing government initiatives for the management and treatment of hemoglobinopathy, which is expected to drive the hemoglobinopathy market growth over the forecast period. For instance, on February 1 2023, the Government of India announced to launch a mission to eliminate sickle cell anemia by 2047 in the union budget of FY 2023-24.
Global Hemoglobinopathy Market: Restraint
Failing clinical trials for the treatment of sickle cell anemia
The global hemoglobinopathy market can be hindered by failing of clinical trials for the treatment of sickle cell anemia. For instance, in April 2022, Imara Inc., a clinical-stage biopharmaceutical company, announced that an Imara drug that hits a novel target to treat rare, inherited hemoglobin disorders has failed two mid-stage studies, one for sickle cell disease and the other for beta thalassemia.
Global Hemoglobinopathy Market - Key Players
Major players operating in the global hemoglobinopathy market include Anamol Laboratories Pvt. Ltd., Bio-Rad Laboratories, Inc., PerkinElmer Inc., Zentech, Sebia, Sysmex Europe SE, Nanjing Poclight Biotechnology Co., Ltd, Abbott, Danaher, ReachBio LLC, Shenzhen Mindray Bio-Medical Electronics Co., Ltd., Nexcelom Bioscience LLC., NIHON KOHDEN CORPORATION, Siemens Healthcare GmbH, CRISPR Therapeutics.
*Definition: The hemoglobinopathies are a group of disorders passed down through families (inherited) in which there is abnormal production or structure of the hemoglobin molecule. Sickle cell disease (SCD) is one such blood disorder caused by the abnormal hemoglobin that damages and deforms red blood cells.
Share
Share
About Author
Manisha Vibhute is a consultant with over 5 years of experience in market research and consulting. With a strong understanding of market dynamics, Manisha assists clients in developing effective market access strategies. She helps medical device companies navigate pricing, reimbursement, and regulatory pathways to ensure successful product launches.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients