Global healthcare CMO market is estimated to be valued at USD 196.61 Bn in 2025 and is expected to reach USD 520.13 Bn by 2032, exhibiting a compound annual growth rate (CAGR) of 14.9% from 2025 to 2032.
Discover market dynamics shaping the industry: Download Free Sample
Growing expenditure on research and development and increasing outsourcing of clinical trial activities by pharmaceutical and biotechnology companies can boost demand for healthcare CMO services. Furthermore, growing generics market and emergence of biopharmaceuticals have prompted biopharma companies to rely on contract manufacturing organizations for their large-scale commercial manufacturing needs. However, pricing pressure faced by CMOs due to intensifying competition and regulatory challenges associated with manufacturing cell and gene therapies can hamper the market growth during the forecast period.
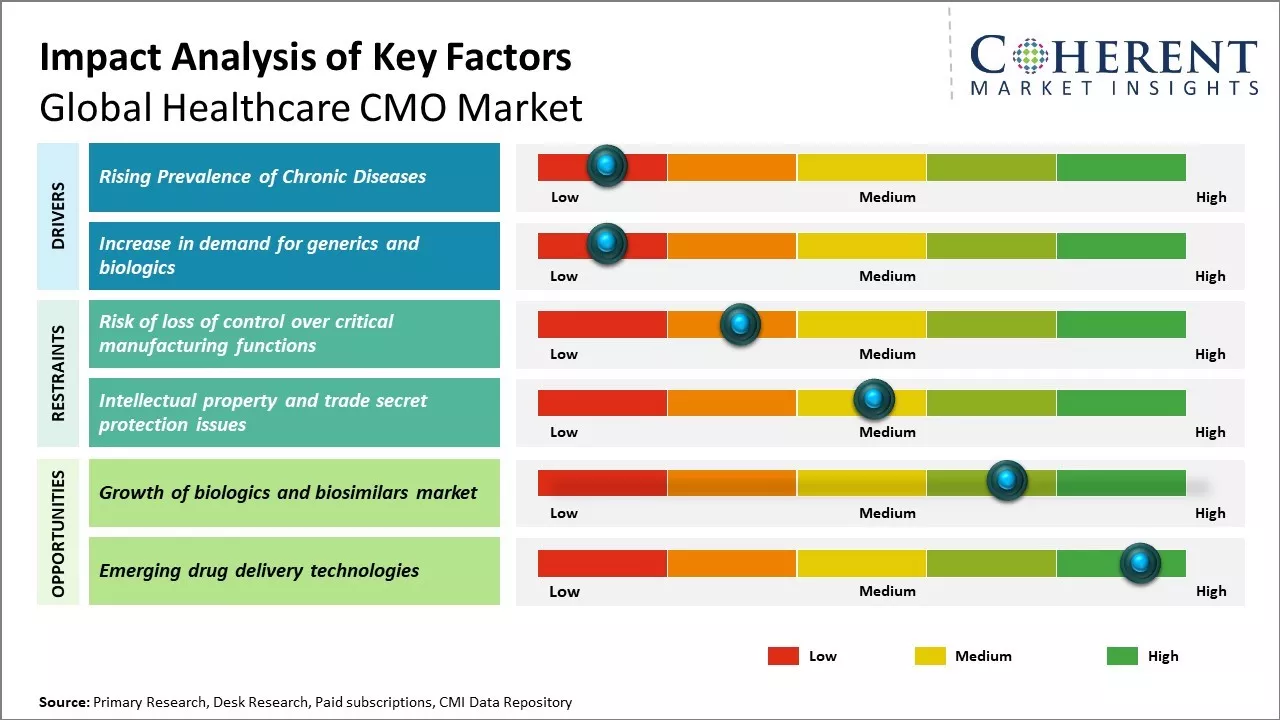
Market Driver: Rising Prevalence of Chronic Diseases
Rising prevalence of chronic diseases such as cancer, diabetes, cardiovascular diseases can drive the market growth. These diseases often require prolonged treatment therapies and complex medical procedures. According to WHO data, chronic diseases are responsible for over 70% of global deaths each year. The increasing number of patients dealing with complex chronic conditions has put substantial strain on healthcare systems worldwide. Pharmaceutical companies are facing escalating R&D costs for creating advanced therapies. To address this, they are outsourcing non-core functions such as clinical trials, manufacturing, and logistics to specialized Contract Manufacturing Organizations (CMOs). This strategy allows drug makers to concentrate on their core competencies, thereby driving up the demand for CMO services.
Get actionable strategies to beat competition: Download Free Sample
Increase in demand for generics and biologics
Rising demand for generic and biosimilar drugs can drive the market growth. As patent cliffs of many blockbuster drugs concluded and patients looked for more affordable treatment options, generics offered a cost-effective solution. These contain the same active pharmaceutical ingredients as their brand-name counterparts but are often priced 80-85% lower than branded drugs. This has driven several pharmaceutical companies to turn to contract manufacturing organizations (CMOs) to help produce the increasing volumes of generics required to meet the growing needs. CMOs allow drug makers to focus on their core competencies of research and development while outsourcing production activities. This provides flexibility and capacity needed to scale up or down as market demand fluctuates for different generic products. According to the World Health Organization, as of 2020, over half the people globally do not have full access to essential health services. The pandemic further exacerbated this situation, stressing the importance of enhancing healthcare access. The development of biologics, including vaccines, to treat rare diseases has also increased in the past couple of years. However, high costs remain a significant barrier. The production of affordable biosimilars by CMOs can expand treatment to more patients. There has been rising need for generics and due to aging populations and growing prevalence of chronic diseases.
Key Takeaways from Analyst:
Global healthcare CMO market growth is driven by rising investments from big pharma companies in drug development as these look to outsource non-core activities. Increased drug development and clinical trial outsourcing to reduce costs can also drive the market growth.
North America dominates the market due to large pharmaceutical companies and thriving biotech industry in the U.S. However, Asia Pacific is likely to emerge as the fastest growing region due to lower manufacturing costs and high-quality standards. Countries like China and India offer significant potential due to improving healthcare infrastructure and regulatory environment.
Capacity constraints and quality issues pose challenges for CMOs to take on new business. Long approval timelines for facility expansion can hamper the market growth. Manufacturing complex drugs can also hamper the market growth due to high technical requirements.
Providing specialized services across the drug development value chain from early stage to commercialization can offer growth opportunities. CMOs expanding into new therapy areas like cell and gene therapy can witness growth. Those providing integrated solutions through mergers and acquisitions are well-positioned to take advantage of the projected growth. Outsourcing of packaging and labelling functions can offer new growth opportunities.
Market Challenges: Risk of loss of control over critical manufacturing functions
Risk of loss of control over critical manufacturing functions can hamper the global healthcare CMO market growth. When companies outsource their manufacturing operations to contract manufacturing organizations, these lose direct control over certain important processes. This loss of control can potentially compromise the quality, consistency and security of supply of products. Manufacturing drugs, medical devices and other healthcare products requires maintaining stringent quality standards and regulatory compliance as even minor issues can risk patient health and safety. Outsourcing to a third party reduces the oversight and management that companies have over their own in-house facilities. Some CMOs may not have the same emphasis on quality management systems or track record of the client company itself. This can introduce the risk of quality deviations, non-compliance issues or other operational disruptions outside the control of product sponsors. Outsourcing manufacturing to a CMO locations the operations overseas in some cases. This geographic separation further reduces the hands-on management and monitoring capabilities of companies. It also increases dependencies on international trade flows and shipping networks. Any delays, disruptions or politico-economic issues in these areas could negatively impact just-in-time manufacturing and supplies. The COVID-19 pandemic highlighted this risk as international transport was significantly impacted during the initial outbreak in 2020. According to the UNCTAD (United Nations Conference on Trade and Development), the pandemic caused a 30-40% decline in volumes of goods traded globally. Many businesses faced severe shortages of components and logistical difficulties. These supply chain issues increased concerns around overly relying on globally distributed manufacturing networks with reduced oversight.
Market Opportunities: Growth of biologics and biosimilars market
The rise of biologics and biosimilars can drive the global healthcare CMO market growth. Biologics are large, complex molecules produced using biotechnology, and have revolutionized the treatment of various diseases. However, the development and manufacturing of biologics is an intricate process which requires specialized expertise and facilities. This complexity has driven significant demand for outsourcing to contract manufacturing organizations (CMOs) that have the specialized capabilities and infrastructure to support biologics development and production. As biologics go off-patent in the coming years, the market for biosimilars - which are biologics that are highly similar to an existing FDA-approved biologic - is also expected to grow substantially. In 2020, the World Health Organization estimated that biosimilars could help achieve considerable cost savings for both patients and healthcare systems. As more biosimilars enter the market, these will boost demand for CMO services to facilitate their development and manufacturing at commercial scale. Both small biotech companies developing biosimilars as well as large biopharma firms expanding into this area will rely heavily on CMOs to assume part or all of the manufacturing responsibilities. The heightened reliance on CMOs is evidenced in recent facility expansions and partnerships. For example, in 2022, the U.S. FDA approved a new biologics manufacturing plant in Ireland that will serve as a dedicated vaccine production facility for a major pharmaceutical company. Top 10 global CMO announced plans in 2021 to double its biomanufacturing capacity in Europe to meet burgeoning client demands in both biologics and vaccines. These significant investments reflect the optimism of both CMOs and their biopharma partners regarding the promise of the biosimilars segment and its potential to spur increased outsourcing activities.
Discover high revenue pocket segments and roadmap to it: Download Free Sample
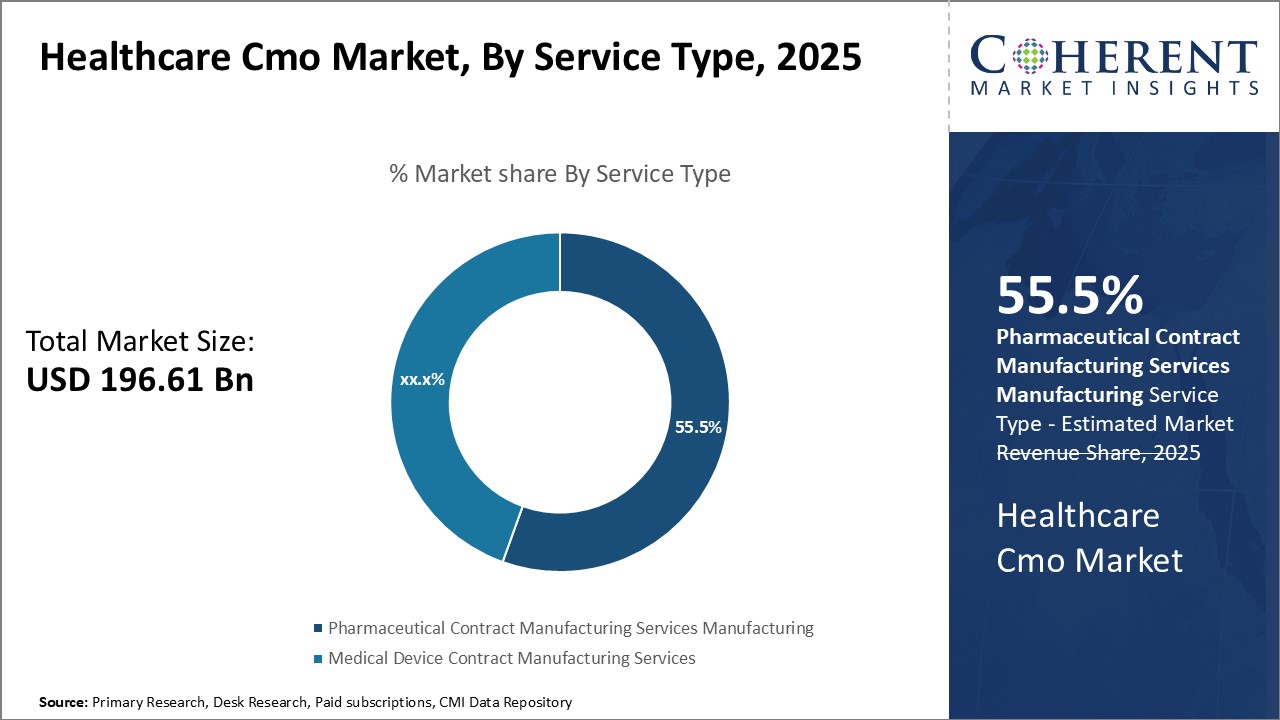
In terms of Service Type, Pharmaceutical Contract Manufacturing Services segment contributes the highest share of the market, owing to increasing outsourcing needs of pharmaceutical companies.
By service type, pharmaceutical contract manufacturing services segment is estimated to hold the highest market share of 55.5% in 2025, primarily due to growing need for outsourcing among pharmaceutical manufacturers. As research and development activities in the pharmaceutical industry continue to rise in complexity, the focus on core competencies has increased outsourcing. Manufacturers prefer contract manufacturing organizations to handle non-core activities so that these can concentrate resources on drug discovery and clinical trials. This boosts demand for Pharmaceutical CMOs. The ability of CMOs to ensure quality and adherence to compliance standards can also drive the segment growth. As regulations around manufacturing practices have become more stringent, especially in developed markets, CMOs provide the expertise, facilities and processes to meet all applicable quality and regulatory guidelines. This provides assurance to drug sponsors and helps accelerate their product pipelines. CMOs also benefit from economies of scale due to their larger facilities and diversified client bases. This allows them to produce drugs at competitive costs. Growing workload of generic drugs boosts greater reliance on CMOs. With many blockbuster drug patents expiring, generic manufacturers are ramping up production volumes significantly. Partnering with well-established CMOs helps them to rapidly scale up operations in a compliant manner.
Need a Different Region or Segment? Download Free Sample
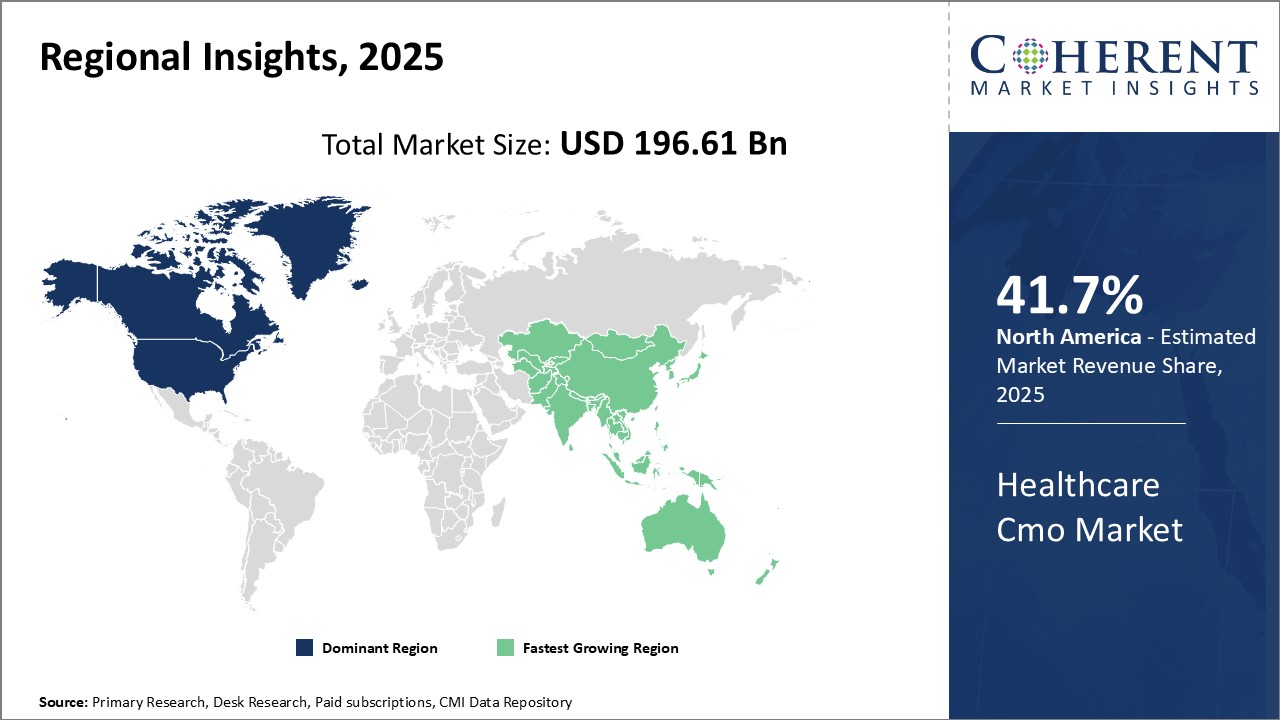
North America dominates the global healthcare CMO market with an estimated market share of 41.7% in 2025, due to presence of many large pharmaceutical and medical device companies driving outsourcing activities. North America also has a highly developed healthcare infrastructure and growing in-house R&D expenditure of pharma firms that leads to increasing product development and clinical research opportunities for CMOs. Furthermore, the region is characterized by a favorable regulatory environment laid out by the U.S.FDA that helps CMOs build credibility to serve large multinational clients. However, pricing pressure to reduce production costs has encouraged some manufacturers to evaluate alternatives in other geographic regions.
Asia Pacific has emerged as the fastest growing regional market for healthcare CMO. Several factors are attributed to its rapid growth including growing pharmaceutical manufacturing capabilities, low production costs, and improving regulatory infrastructure in many countries. The presence of established generic drug manufacturing industries in India and China presents enormous potential for CMOs to offer integrated services to their clients. Geographical proximity to these markets also provides Asia Pacific CMOs an advantage over their North American counterparts in terms of responsiveness and turnaround time. India and China enjoy immense investments from international drug makers who have set up sophisticated manufacturing plants catering to both domestic as well as exports markets. Their governments have also implemented various incentives to attract investments that help CMOs enhance their technical expertise. This has led to rising penetration of several Asia Pacific-based CMOs in developed regions through outsourced studies and contract filling orders. While their costs are significantly lower than North America, client confidence in quality and compliance is growing.
Healthcare CMO Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 196.61 Bn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 14.9% | 2032 Value Projection: | USD 520.13 Bn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
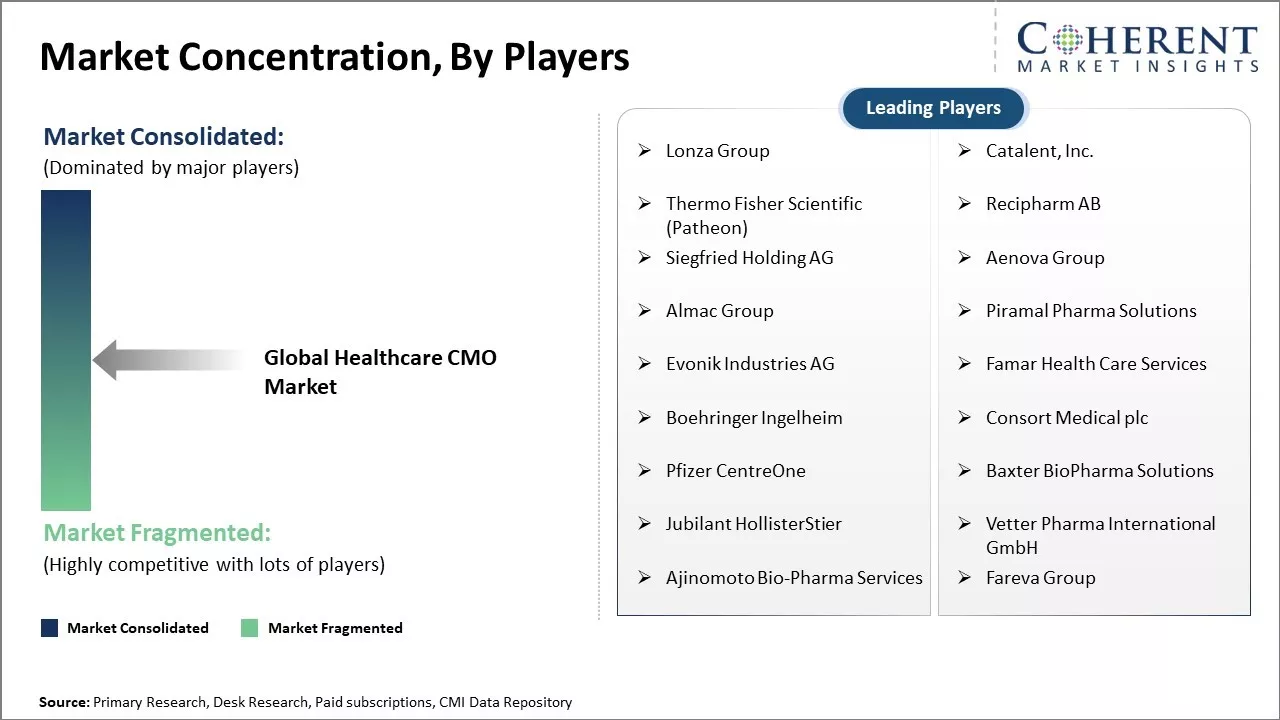
| Companies covered: |
Lonza Group, Catalent, Inc., Thermo Fisher Scientific (Patheon), Recipharm AB, Siegfried Holding AG, Aenova Group, Almac Group, Piramal Pharma Solutions, Evonik Industries AG, Famar Health Care Services, Boehringer Ingelheim, Consort Medical plc, Pfizer CentreOne, Baxter BioPharma Solutions, Jubilant HollisterStier, Vetter Pharma International GmbH, Ajinomoto Bio-Pharma Services, Fareva Group |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
*Definition: Global Healthcare CMO Market involves contract manufacturing organizations that provide clinical trial material production, commercial manufacturing, packaging and distribution services to pharmaceutical, biotech and medical device companies. CMOs help speed products to market and focus internal resources on core competencies like R&D. The global healthcare CMO industry handles sterile and non-sterile pharmaceutical manufacturing as well as medical device manufacturing, assembly and packaging for customers worldwide. CMOs aid medical product development and commercialization through flexible, scalable outsourcing solutions.
Share
Share
About Author
Komal Dighe is a Management Consultant with over 8 years of experience in market research and consulting. She excels in managing and delivering high-quality insights and solutions in Health-tech Consulting reports. Her expertise encompasses conducting both primary and secondary research, effectively addressing client requirements, and excelling in market estimation and forecast. Her comprehensive approach ensures that clients receive thorough and accurate analyses, enabling them to make informed decisions and capitalize on market opportunities.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients