Global Monochrome Medical Monitor Market Size and Forecast – 2026 – 2033
The Global Monochrome Medical Monitor Market size is estimated to be valued at USD 620 million in 2026 and is expected to reach USD 1,000 million by 2033, exhibiting a compound annual growth rate (CAGR) of 7.2% from 2026 to 2033.
Global Global Monochrome Medical Monitor Market Overview
Monochrome medical monitors are specialized display products used in medical imaging and diagnostic environments. These monitors provide high grayscale accuracy and contrast resolution for viewing X-rays, CT scans, MRIs, and other diagnostic images. They are widely used in radiology departments, diagnostic centers, and hospitals. Monochrome medical monitors are designed to meet strict medical display standards. Image clarity, calibration stability, and diagnostic accuracy are core product attributes.
Key Takeaways
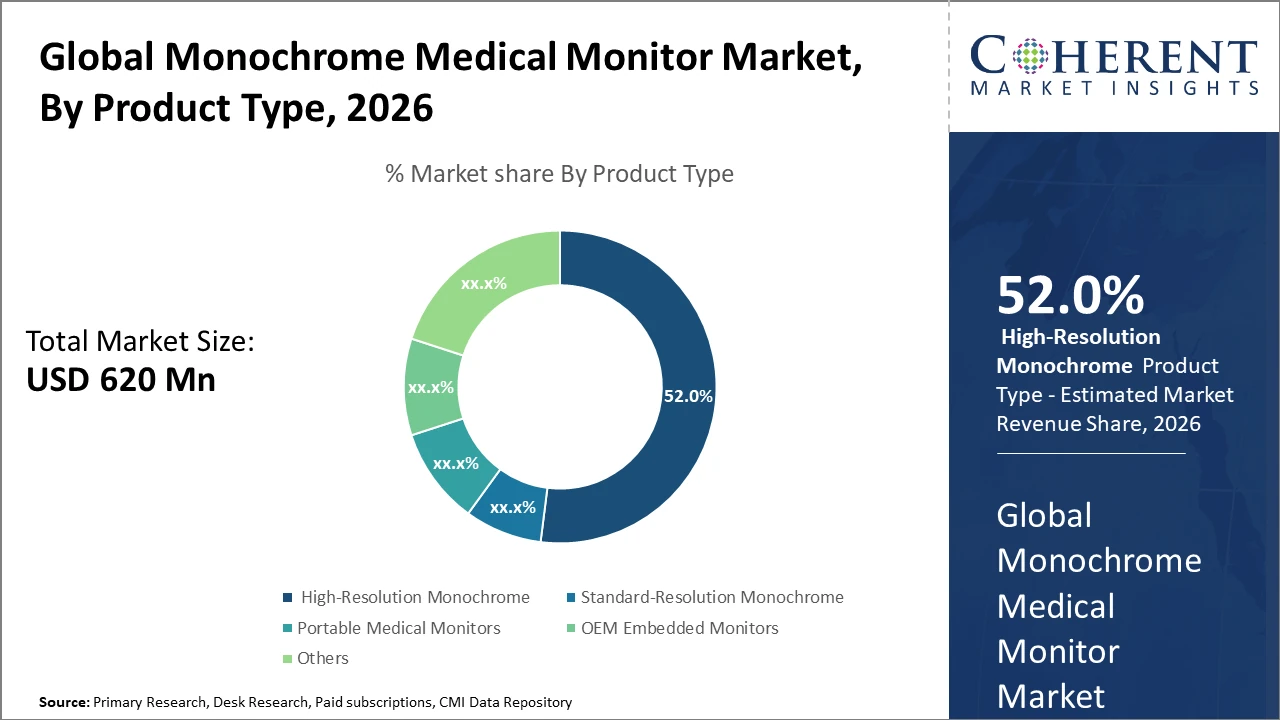
The High-Resolution Monochrome segment retains dominance with over 52% industry share, driven by its superior imaging quality essential for cardiology and radiology applications.
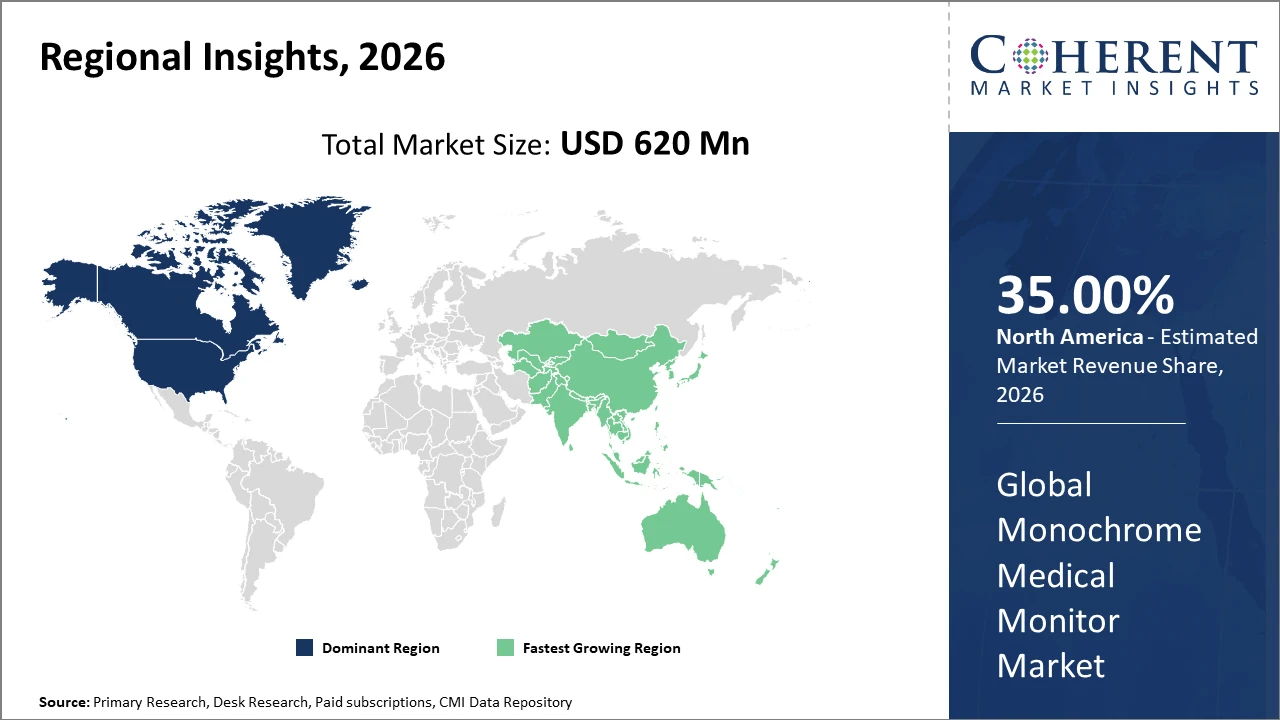
Asia Pacific emerges as the fastest-growing region, recording a significant CAGR backed by increasing healthcare spending and favorable government policies in countries such as China and India.
North America holds the largest market share by 35% revenue contribution, supported by advanced healthcare infrastructure and the presence of major market players.
The rising demand for portable monitors in Latin America indicates a growing opportunity for investors targeting mobile healthcare solutions.
Global Monochrome Medical Monitor Market Segmentation Analysis
To learn more about this report, Download Free Sample
Global Monochrome Medical Monitor Market Insights, By Product Type
High-Resolution Monochrome monitors lead due to their superior image quality required for critical diagnostics like radiology and cardiology, which demand detailed grayscale differentiation. This segment is supported by widespread hospital adoption, where image clarity directly impacts diagnostic outcomes. The fastest growing subsegment is Portable Medical Monitors, propelled by increasing demand for mobile healthcare solutions and emergency medical applications, where portability and durability are critical.
Global Monochrome Medical Monitor Market Insights, By Application
Cardiovascular Imaging is maintaining dominance in the market share due to the rising prevalence of cardiovascular diseases worldwide, requiring advanced diagnostic imaging. Accurate grayscale rendering is essential for these monitors to discern subtle clinical signs, boosting demand within this segment. Endoscopy stands as the fastest-growing subsegment, driven by increasing minimally invasive procedures and the need for high-resolution displays capable of rendering fine details in real-time. Surgeons rely heavily on these monitors for precision during procedures, triggering heightened investments, especially in North America and Europe. Ultrasound Imaging comprises a significant share, utilized broadly for diagnostic assessments across various specialties.
Global Monochrome Medical Monitor Market Insights, By End-User
Hospitals dominate the market share due to their substantial investments in advanced medical equipment and continuous upgrade cycles aimed at enhancing diagnostic accuracy and patient outcomes. Diagnostic Centers are the fastest-growing subsegment as they proliferate globally to meet rising outpatient imaging demands, offering specialized services often requiring cutting-edge monochrome monitors. The shift towards outpatient diagnostics and cost-effective healthcare models fuels this trend.
Global Monochrome Medical Monitor Market Trends
Recent market trends highlight a strong push towards high-precision imaging through technological enhancements such as 12-bit grayscale rendering and improved luminance uniformity, critical for diagnostic accuracy.
For example, in 2024, hospitals utilizing next-gen monochrome monitors reported up to a 10% improvement in diagnostic confidence scores.
Additionally, the movement towards modular and portable systems continues, addressing on-the-go clinical demands, as seen in emergency medical services across North America and Europe.
The integration of AI-driven image processing also facilitates faster and more accurate interpretations, reducing radiologists' workload.
Global Monochrome Medical Monitor Market Insights, By Geography
To learn more about this report, Download Free Sample
North America Global Monochrome Medical Monitor Market Analysis and Trends
In North America, the dominance in the Monochrome Medical Monitor market is attributed to its vast healthcare infrastructure, high adoption of advanced medical imaging technologies, and stringent quality standards. The U.S., contributing a majority share within this region, drives significant market revenue through demand in hospitals and outpatient centers. The presence of major manufacturing units and supportive reimbursement policies further consolidates the region’s leadership.
Asia Pacific Global Monochrome Medical Monitor Market Analysis and Trends
Meanwhile, Asia Pacific exhibits the fastest growth, with a CAGR exceeding 8%, fueled by expanding healthcare budgets, rising government initiatives to upgrade medical facilities, and increasing patient awareness. China and India stand out with rapid hospital expansions and the installation of advanced imaging systems. The growing domestic manufacturing capabilities and supportive trade policies also bolster market penetration and revenue.
Global Monochrome Medical Monitor Market Outlook for Key Countries
USA Global Monochrome Medical Monitor Market Analysis and Trends
The USA's market is heavily influenced by continuous improvements in healthcare IT infrastructure and increasing demand for precision diagnostics. Leading medical centers, such as those in Massachusetts and California, have spearheaded the adoption of advanced monochrome monitors offering enhanced grayscale accuracy, reflected in a 14% year-on-year increase in procurement as of 2024. The government's emphasis on patient safety and quality imaging standards under CMS regulations has further propelled market growth. Market players leverage these trends by engaging in R&D, collaborations, and customized solutions to maintain a competitive edge.
India Global Monochrome Medical Monitor Market Analysis and Trends
India’s market is expanding rapidly due to significant investments in healthcare infrastructure, including government-funded modernization of district hospitals and diagnostic centers. The national telemedicine initiative fostered wider deployment of portable monochrome monitors, particularly in rural health settings, boosting demand by approximately 18% in 2024 alone. Local manufacturers alongside international entrants are tapping into this growth by offering competitively priced devices tailored to regional requirements. Favorable regulatory reforms and rising private sector healthcare investments ensure sustained market momentum.
Analyst Opinion
Supply-side dynamics reveal a steady increase in production capacity across leading manufacturing hubs, especially in North America and Asia Pacific, with capacity expansions reported to have increased by over 15% in 2024. This is indicative of manufacturers’ confidence in the growing market demand and aligns with increased procurement from diagnostic centers worldwide. For instance, a noted facility in Texas expanded output by 18% to meet escalating orders.
Pricing strategies have shown moderate stabilization despite fluctuating raw material costs, helped by long-term contractual agreements with key suppliers. Notably, unit prices for high-resolution monochrome monitors rose marginally by 3% in 2025, reflecting inflation and enhanced feature integration, yet remaining competitive for healthcare providers.
Demand-side analysis highlights broad application in critical imaging procedures, where monochrome monitors are preferred due to superior grayscale rendering. Usage in cardiology and radiology has surged, with hospital acquisitions of such displays increasing by approximately 12% between 2023 and 2025, driven by the need for acute imaging accuracy.
Export activities have grown substantially, with Asia Pacific emerging as a major supplier to Latin America and parts of Europe. Exports of monochrome medical monitors from key hubs in South Korea and China rose by 20% in 2024, underscoring the global nature of market revenue streams and the strategic importance of cross-border trade partnerships.
Market Scope
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2025 | Market Size in 2026: | USD 620 million |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2026 To 2033 |
| Forecast Period 2026 to 2033 CAGR: | 7.2% | 2033 Value Projection: | USD 1.0 billion |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: | JVC Kenwood Corporation, ViewSonic Corporation, Siemens Healthineers AG, Toshiba Corporation, Philips Healthcare, Hitachi Ltd., Fujifilm Holdings Corporation, NDS Surgical Imaging, Shimadzu Corporation, Carestream Health, Inc. | ||
| Growth Drivers: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Global Monochrome Medical Monitor Market Growth Factors
Increasing adoption of advanced diagnostic procedures in hospitals, such as endoscopy and cardiovascular imaging, demands high-accuracy monochrome monitors capable of precise grayscale presentation. Recent hospital networks in the U.S. reported a 14% increase in procurement of such monitors in 2025. Government initiatives enhancing healthcare infrastructure in emerging economies have accelerated market growth; for instance, investments exceeding USD 500 million in healthcare modernization in India during 2024 positively influenced regional industry size. The rising prevalence of chronic diseases globally has led to expanding diagnostic imaging requirements, consequently increasing application areas and boosting overall market revenue.
Global Monochrome Medical Monitor Market Development
In October 2024, Barco introduced the Coronis OneLook, a flagship 32-megapixel diagnostic display developed specifically for breast imaging and mammography. The large-format display enables radiologists to view full-field mammography and tomosynthesis images at native resolution without zooming or panning, improving reading efficiency and diagnostic confidence. This launch reinforces Barco’s focus on high-resolution visualization solutions for advanced radiology workflows.
In May 2025, EIZO launched the RadiForce RX570, a 5-megapixel (21.3-inch) color diagnostic monitor optimized for monochrome-intensive applications such as mammography and digital breast tomosynthesis. The display delivers high luminance stability, precise grayscale reproduction, and color accuracy, supporting subtle lesion detection and consistent diagnostic performance. The RX570 expands EIZO’s RadiForce portfolio for breast imaging and multi-modality diagnostic environments.
Key Players
Leading Companies of the Market
JVC Kenwood Corporation
ViewSonic Corporation
Siemens Healthineers AG
Toshiba Corporation
Philips Healthcare
Hitachi Ltd.
Fujifilm Holdings Corporation
NDS Surgical Imaging
Shimadzu Corporation
Carestream Health, Inc.
Several market players have adopted distinctive growth strategies to strengthen their position. For example, Barco NV entered into strategic partnerships with global healthcare providers, improving customer access and driving a 10% increment in revenue from 2023 to 2025. Similarly, Eizo Corporation focused on R&D investments to develop advanced monochrome monitors with enhanced ergonomic designs, resulting in a 15% market share gain in the Asia Pacific region by early 2025. Another leading company, LG Display, leveraged vertical integration to reduce production costs, improving margins despite volatile component prices.
Global Monochrome Medical Monitor Market Future Outlook
The future of the global monochrome medical monitor market will be driven by increasing diagnostic imaging demand, aging populations, and expanding healthcare infrastructure, particularly in emerging economies. Replacement demand from existing installations will remain a steady revenue contributor. Advances in display technology, energy efficiency, and ergonomic design will further enhance value propositions. While color imaging will continue to grow, monochrome monitors are expected to remain indispensable for high-precision diagnostic applications, ensuring sustained relevance in the global healthcare imaging ecosystem.
Global Monochrome Medical Monitor Market Historical Analysis
The global monochrome medical monitor market evolved alongside the expansion of diagnostic imaging technologies such as X-ray, CT, and mammography. Early monitors offered limited grayscale accuracy and resolution, which constrained diagnostic confidence. As regulatory standards for medical imaging tightened, manufacturers invested heavily in display calibration, luminance stability, and image consistency. Adoption increased steadily in radiology departments, diagnostic centers, and hospitals as imaging volumes grew worldwide. Despite the emergence of color displays, monochrome monitors retained strong demand due to their superior grayscale performance and diagnostic reliability.
Sources
Primary Research Interviews:
Radiologists
Imaging technicians
PACS administrators
Display manufacturers
Databases:
FDA Imaging Devices
WHO Diagnostic Imaging Data
OECD Health Equipment Data
Magazines:
Diagnostic Imaging
Imaging Technology News
Radiology Business
Medical Imaging Review
HealthTech Magazine
Journals:
Radiology
European Radiology
Journal of Digital Imaging
Academic Radiology
Medical Physics
Newspapers:
Financial Times (Healthcare)
Reuters Medical Technology
The Wall Street Journal (Health)
Bloomberg Technology
The Guardian (Science)
Associations:
Radiological Society of North America
European Society of Radiology
Society for Imaging Informatics in Medicine
American College of Radiology
International Society for Optics and Photonics
Share
Share
About Author
Manisha Vibhute is a consultant with over 5 years of experience in market research and consulting. With a strong understanding of market dynamics, Manisha assists clients in developing effective market access strategies. She helps medical device companies navigate pricing, reimbursement, and regulatory pathways to ensure successful product launches.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients