3D printing, also referred as additive manufacturing, is used to manufacture several medical devices in the healthcare sector such as orthopedic and cranial implants, surgical instruments, dental restorations such as crowns, and external prosthetics.
The global 3D printed medical devices market is estimated to account for US$ 1,737.5 Mn in terms of value by the end of 2027.
Global 3D Printed Medical Devices Market: Drivers
High prevalence of periodontitis, diabetes-related gangrene, osteoarthritis, bone-dental diseases, and peripheral vascular disease is expected to boost growth of the global 3D printed medical devices market. For instance, in April 2018, researchers at Sri Ramachandra University, India, reported 42.3% prevalence of periodontal disease in southern India.
Moreover, increasing number of accidents is also expected to aid in growth of the market. For instance, according to 2018 Fatal Motor Vehicle Crashes: Overview by National Highway Traffic Safety Administration, a U.S. federal agency, 10,511 deaths were registered in the U.S. in 2018.
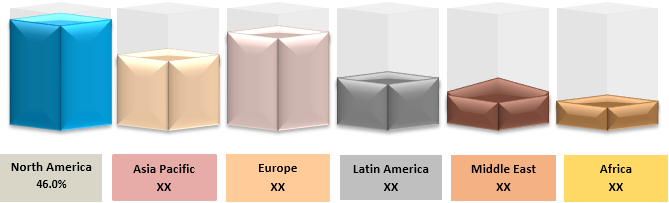
North America region held dominant position in the global 3D printed medical devices market in 2019, accounting for 46.0% share in terms of volume, followed by Europe.
Figure 1. Global 3D Printed Medical Devices Market Value (US$ Mn), by Region, 2019
To learn more about this report, Download Free Sample
Global 3D Printed Medical Devices Market: Restraints
Less number of materials are suitable for 3D printing of medical devices, which is expected to hinder growth of the market. Materials used for 3D printing include, resins, plastics, and a few metals.
Moreover, the small size of 3D printers limits the size of the end product, which may not be feasible for production of parts of large industrial machines. This in turn is expected to hamper growth of the market.
Global 3D Printed Medical Devices Market: Opportunities
Increasing R&D in biocompatible materials and devices is expected to offer lucrative growth opportunities for players in the global 3D printed medical devices market. For instance, in February 2019, researchers from Simon Fraser University reported development of eco?friendly 3D?printed disposable sensor devices with smart 3D form factor using biocompatible cellulose composites.
Increasing adoption of bio-printed animal and human cells in drug testing is also expected to aid in growth of the market. For instance, India-based Next Big Innovation Lab uses 3D bioprinting for developing animal and human cells for testing cosmetics and drugs.

To learn more about this report, Download Free Sample
PolyJet / InkJet 3D Printing segment in the global 3D printed medical devices market was valued at US$ 89.3 Mn in 2019 and is expected to reach US$ 348.1 Mn by 2027 at a CAGR of 18.4% during the forecast period.
Market Trends/Key Takeaways
Major players in the market are focused on developing porous 3D printed PEEK porous medical implants. For instance, in January 2020, FossiLabs, LLC, a US-based medical 3D printing start-up, launched its FFF 3D printed bone-like scaffolding structures using a porous PEEK material.
The market is also witnessing collaboration and partnership activities among players. For instance, in June 2019, PrinterPrezz, the U.S.-based medical 3D printing service bureau, partnered with UCSF Surgical Innovations to develop new 3d printed medical devices.
Regulations
North America
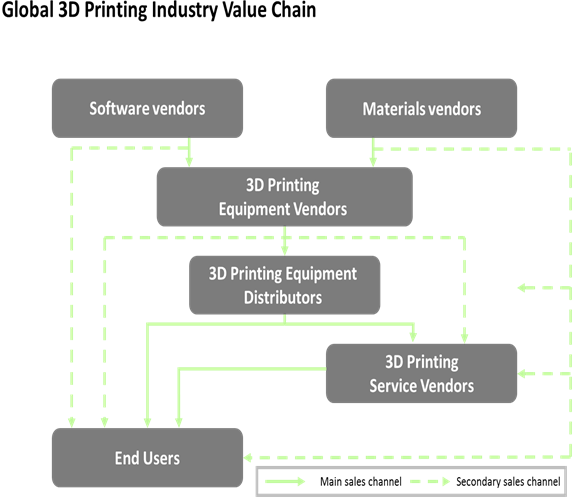
Value Chain Analysis (Description or image)
To learn more about this report, Download Free Sample
Global 3D Printed Medical Devices Market: Competitive Landscape
Major players operating in the global 3D printed medical devices market include, 3D Systems, Inc., Arcam AB, Stratasys Ltd., FabRx Ltd., EOS GmbH Electro Optical Systems, EnvisionTEC, Cyfuse Biomedical K.K., Bio3D Technologies, PrinterPrezz, Carima, Nexxt Spine, and Aurora Labs.
Global 3D Printed Medical Devices Market: Key Developments
Key players in the market are focused on expanding their capabilities to enhance their market share. For instance, in April 2019, Nexxt Spine, the U.S.-based medical device manufacturer, expanded its metal 3D printing capabilities with the installation of two Concept Laser Mlab 100R systems from GE Additive.
Key players in the market are focused on product development to expand their product portfolio. For instance, in February 2019, Aurora Labs, the Australia-based metal 3D printer manufacturer, partnered with the University of Western Australia (UWA) and Royal Perth Hospital (RPH) to develop designs, specifications, and parameters for the 3D printing of titanium medical implants.
Share
Share
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients