Glioblastoma is a type of brain tumor that arises from the glial or supportive brain tissue called ‘glioma’. Glioblastoma multiforme (abbreviated as GBM) is an aggressive malignant brain tumor that has a very poor prognosis. The commercially available treatment option for this condition faces many challenges, including the crossing of blood brain barrier, which are limiting the concentration of drugs at the tumor site. Key companies in this market are actively focused on research & development activities to develop, and commercialize novel drugs for the treatment of such condition.
According to the Journal of Neurosurgery in 2018, advancements in the last few decades have not significantly improved the overall survival of patients with recurrent glioblastoma multiforme (GBM) disease. Furthermore, brief discussions related to current novel efforts in clinical and basic research are included in the journal. As per these brief discussions by medical experts, it is concluded that a subset of recurrent GBM patients may benefit from maximum treatment efforts although this disease remains a fatal disease.
Increasing incidence of brain tumors is expected to drive growth of the global recurrent glioblastoma multiforme treatment market during the forecast period. As per the National Foundation for Cancer Research, GBM is the deadliest type of brain cancer, accounting for 45% of all malignant brain tumors. Furthermore, according to the article published by National Brain Tumor Society, over 10% of people with brain tumors are at high risk of developing glioblastomas.
Recurrent Glioblastoma Multiforme Treatment Market - Impact of Coronavirus (Covid-19) Pandemic
The treatment for recurrent glioblastoma multiforme and other cancers are immensely impacted due to the sudden outbreak of COVID-19 across the globe. Patients are unable to access health and surgery centers for their respective radiotherapy, chemotherapy, and health monitoring. This pandemic has also impacted the global pharmaceutical supply chain, thereby hindering the accessibility and availability of treatment for patients with recurrent glioblastoma multiforme.
The all-inclusive version of the report will include the impact of COVID-19 and the probable changes in the future outlook of the industry, by taking into account the technological, social, political, and economical parameters.
The global recurrent glioblastoma multiforme treatment market is estimated to be valued at US$ 369.6 million in 2020 and is expected to exhibit a CAGR of 6.14% during the forecast period (2020-2027).
Figure 1: Recurrent Glioblastoma Multiforme Treatment Market Share (%) Analysis, By Type, 2020
To learn more about this report, Download Free Sample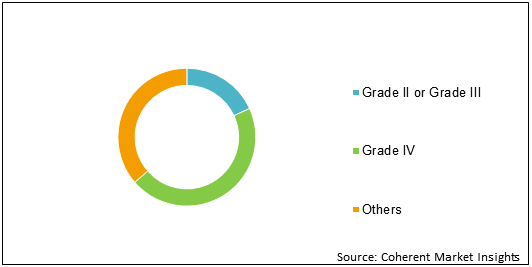
New drug approvals and launches for GBM treatme nt is expected to boost growth of the global recurrent glioblastoma multiforme treatment market
In January 2020, Pfizer launched ‘Zirabev’ in the U.S., which is a bevacizumab biosimilar of Avastin, approved for the treatment of recurrent glioblastoma multiforme. The biosimilar was approved by FDA in June 2019 for the treatment of metastatic or recurrent NSCLC, metastatic colorectal cancer, recurrent glioblastoma multiforme, recurrent/persistent or metastatic cervical cancer, and metastatic renal cell carcinoma.
Furthermore, key players in the industry are involved in joint ventures and collaborations to develop and commercialize novel drugs, in order to strengthen their position in the global recurrent glioblastoma multiforme treatment market. For instance, in May 2017, DelMar Pharmaceuticals formalized the collaboration agreement with PRA Health Sciences to conduct phase 3 trials of VAL-083 for the treatment of recurrent GBM. PRA Health Sciences is a contract research organization (CRO) that provides outsourced clinical development services to the pharmaceutical and biotechnology industries.
In November 2019, the U.S. FDA accepted Samsung’s BLA application for SB8 bevacizumab, a biosimilar of Avastin. If this gets approval, this biosimilar will be commercialized in the U.S. market by Merck & Co., Inc., which has also been Samsung’s partner on its biosimilar of infliximab – Renflexis.
Recurrent Glioblastoma Multiforme Treatment Market Restraints
However, lack of effective therapies and the inability of these therapies to effectively prevent tumor recurrence are the major factors, expected to hamper growth of the recurrent glioblastoma multiforme treatment market.
Recurrent Glioblastoma Multiforme Treatment Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2019 | Market Size in 2019: | US$ 369.6 Mn |
| Historical Data for: | 2017 to 2019 | Forecast Period: | 2020 to 2027 |
| Forecast Period 2020 to 2027 CAGR: | 6.14% | 2027 Value Projection: | US$ 560.9 Mn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
GlaxoSmithKline plc., AstraZeneca, F. Hoffman-La Roche, Ltd., Pfizer, Inc., Merck & Co., Inc., Vascular Biogeneics, AngioChem, Inc., Boehringer Ingelheim GmbH, Bristol-Myers Squibb Company, Boston Biomedical, Inc., Cantex Pharmaceuticals, Inc., Celldex Therapeutics, Inc., Cavion LLC, Coherus BioSciences, Inc., Eisai, Eli Lilly and Company, Cortice Biosciences, Inc., EnGeneIC Ltd., GenSpera, Inc., ERC Belgium SA, Genzyme Corporation, ImmunoCellular Therapeutics, Ltd., and GW Pharmaceuticals Plc. |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Recurrent Glioblastoma Multiforme Treatment Market – Regional Analysis
North America accounted for the largest share in the global recurrent glioblastoma multiforme treatment market in 2019, owing to the presence of major market players, increasing healthcare awareness, and FDA approvals for novel drugs in the market. For instance, in October 2019, Denovo Biopharma, a San Diego-based biotech company, was granted the U.S. Food & Drug Administration (FDA) approval for its phase 2b clinical trial evaluating an analytical combination therapy for the treatment of newly diagnosed glioblastoma multiforme patients. Their DB102 treatment was approved for a study to gauge the efficacy in combination with temozolomide (Temodar) and radiation.
Moreover, the adoption of research & development and collaboration strategies by major players in the North America region are also contributing to growth of the global recurrent glioblastoma multiforme treatment market. For instance, in October 2019, UCLA and Advaxix entered into a research collaboration for glioblastoma multiforme (GBM) immunotherapy products. The aim of Advaxix behind this collaboration was to conduct preclinical studies assessing the Lm technology of UCLA in mouse tumor models of GBM.
Furthermore, Asia Pacific companies are also investing in R&D for novel treatment of GBM. Companies such as Samsung Bioepis, Kazia Therapeutics, and others are involved in research & development of novel drugs for the treatment of recurrent glioblastoma multiforme. For instance, in March 2018, the Australia-based Kazia Therapeutics, a biopharma company, started phase II clinical trials in the U.S. for its drug, GDC-0084. Moreover, a novel vaccine, Rindopepimut, is in last-stage development in Australia and India for first-line GBM treatment.
Figure 2: Recurrent Glioblastoma Multiforme Treatment Market Value (US$ Mn) & Y-o-Y Growth (%), 2017-2027
To learn more about this report, Download Free Sample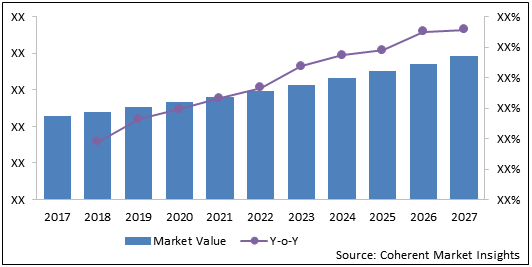
Recurrent Glioblastoma Multiforme Treatment Market - Competitive Landscape
Key players operating in the recurrent glioblastoma multiforme treatment market include GlaxoSmithKline plc., AstraZeneca, F. Hoffman-La Roche, Ltd., Pfizer, Inc., Merck & Co., Inc., Vascular Biogeneics, AngioChem, Inc., B oehringer Ingelheim GmbH, Bristol-Myers Squibb Company, Boston Biomedical, Inc., Cantex Pharmaceuticals, Inc., Celldex Therapeutics, Inc., Cavion LLC, Coherus BioSciences, Inc., Eisai, Eli Lilly and Company, Cortice Biosciences, Inc., EnGeneIC Ltd., GenSpera, Inc., ERC Belgium SA, Genzyme Corporation, ImmunoCellular Therapeutics, Ltd., and GW Pharmaceuticals Plc.
Share
Share
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients