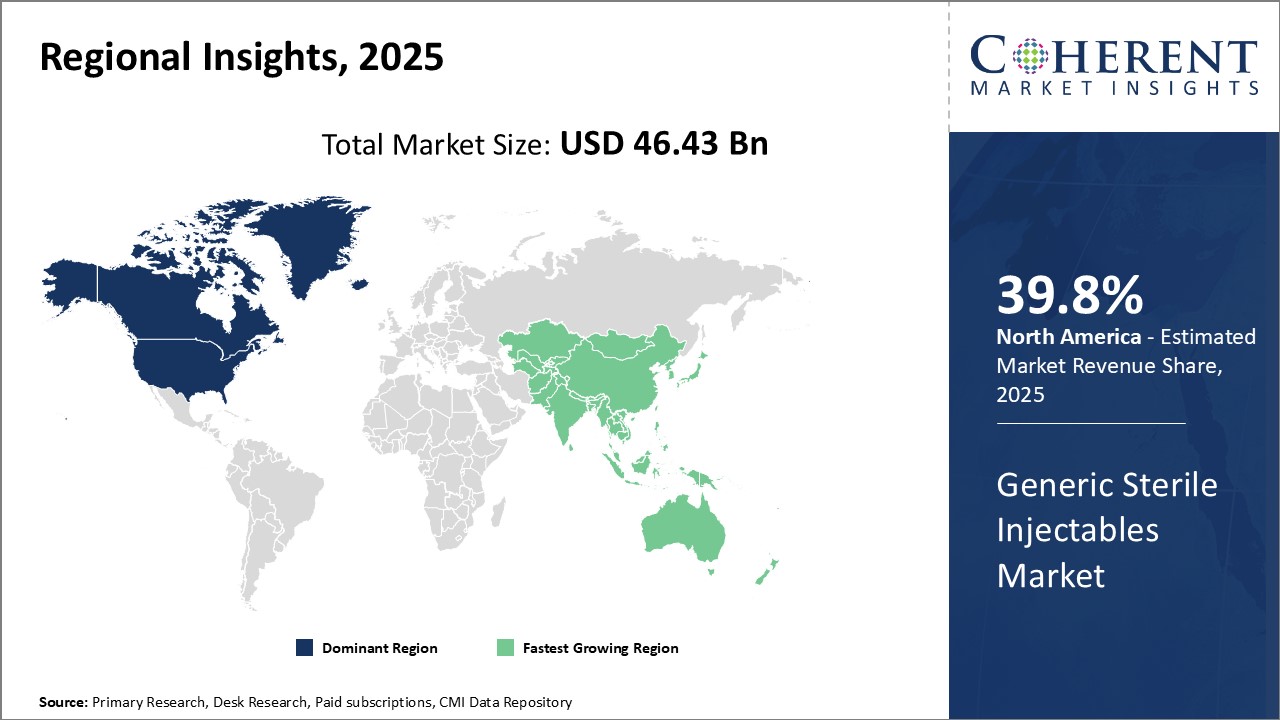
Global Generic Sterile Injectables Market is estimated to be valued at USD 46.43 Bn in 2025 and is expected USD 93.39 Bn by 2032, reflecting a CAGR of 10.5% from 2025 to 2032
Generic sterile injectables are a cost-efficient and bioequivalent option in comparison to name-brand injectables. They are manufactured under strict regulatory frameworks such as the U.S FDA. The increasing worldwide prevalence of chronic illnesses and non-communicable diseases is driving market demand. According to WHO 2023, NCDs account for 74% of deaths worldwide. This is coupled with rising healthcare expenditure, market patent expirations, growing acceptance of biosimilars, and increased patent expiration which fundamentally accelerates the market. In the U.S., approximately 48% of adults possess at least one risk factor for cardiovascular disease, which signifies the importance of affordable, quality treatment alternatives such as generic sterile injectables.
|
Event |
Description and Impact |
|
Technological Advancements |
|
|
Geopolitical Events |
|
|
Mergers and Acquisitions |
|
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
By Type, it seems monoclonal antibodies are set to take over the generic sterile injectable industry, due to their selective action on complicated processes like cancer, autoimmune diseases, and even some clinical infections. This segment is projected to capture 22.2% of the Generic Sterile Injectables Market Demand share in 2025 when the largest market portion is expected, due to biologics like trastuzumab (for metastatic breast cancer) and adalimumab biosimilars (for rheumatoid arthritis) garnering ample support from World Health Organization (WHO) as core therapies. The U.S. FDA Biosimilars Action Plan has more recently enhanced the pace at which other biosimilar monoclonal antibodies are approved, guaranteeing swifter entrance into the market and extensive clinical use. Also, other forms of strategic alliances like those created by the Medicines Patent Pool have increased the availability of biosimilar antibodies in poorer countries, which better aids global access.
Monoclonal antibodies have a preference owing to their clinical correction offering reduced side effects and increased patient outcomes. Their employment in oncology and immunology, as well as institutional policy on the management of sterile injectables in the hospitals, stimulate growth. Monoclonal antibodies for both pharmaceutical companies and healthcare systems offer predictable efficacy alongside numerous available biosimilars, positioning them as the ideal therapeutic class for standardization of biologics. A notable example is Enhertu (trastuzumab deruxtecan), an antibody-drug conjugate developed by AstraZeneca and Daiichi Sankyo. Enhertu combines a monoclonal antibody with a chemotherapy agent, enabling precise delivery of the drug to HER2-positive cancer cells. In a clinical trial involving 524 patients with metastatic breast cancer, Enhertu significantly outperformed the existing standard treatment, Kadcyla, by reducing the risk of disease progression or death by 71.6%. The median progression-free survival for patients treated with Enhertu was 25.1 months, compared to 7.2 months for those on Kadcyla. This targeted approach not only enhances efficacy but also reduces systemic side effects commonly associated with traditional chemotherapy.
By Distribution Channel, hospitals are anticipated to keep the maximum share of the Generic Sterile Injectables Market Forecast in 2025, given the acuteness-of-care feature for these therapies. Chemotherapy and care-intensive antibiotics coupled with emergency biologics need prompt, sterile, and enfolded administration requiring the most monitored reliable delivery point—the hospital. For instance, According to the Centers for Disease Control and Prevention (CDC), proper storage, handling, and administration of injectables are crucial to prevent contamination and ensure patient safety—practices that hospitals are best equipped to maintain due to their infrastructure and trained personnel. For example, the CDC's guidelines for safe injection practices underscore the importance of sterile environments and strict cold-chain management, which hospitals are uniquely capable of implementing consistently. The Joint Commission International (JCI) and even the CDC impose regulatory requirements on proper handling and cold-chain logistics, which bolsters the argument for the hospital’s containment of safe injectable distribution.
On the other hand, retail pharmacies are slowly coming up, particularly in developing countries with growing outpatient services. For example, a component of India’s National Health Mission employs retail pharmacies for better access to insulin and antibiotics in the rural regions. Drug stores, on the other hand, serve as secondary providers for the management of chronic diseases, particularly in areas with weak healthcare systems. Programs such as Uganda's Accredited Drug Dispensing Outlets (ADDOs) illustrate how these channels can be utilized to provide regulated access to injectables like antiretrovirals and TB drugs. However, given the high degree of complexity and time-sensitivity involved in many of the sterile injectable therapies, their provision will continue to rest with hospitals.
By Application, it is estimated that oncology will be the fastest growing segment in the Generic Sterile Injectables Market Growth by 2025 due to the increasing worldwide cancer cases and dependence on biologics. According to IARC international agency for research on cancer, global cancer cases are expected to increase by 47% by 2040 which further emphasizes the need for effective cancer therapies. Trastuzumab biosimilars are now being implemented in regional programs; PAHO encourages the use of trastuzumab in Latin America for breast cancer addressing the survival rates. For instance, The Pan American Health Organization (PAHO) has advocated for the expanded use of trastuzumab to improve survival outcomes in HER2-positive breast cancer patients. In 2021, PAHO included trastuzumab in its Strategic Fund to improve access to quality-assured essential medicines across member states, especially in low- and middle-income regions of Latin America
Diabetes still remains an important application area with the increasing prevalence of metabolic disorders. The WHO Global Diabetes Compact strives for 80% of the population diagnosed with diabetes to have access to low-cost insulin by 2030 which highlights generic insulin and GLP-1 analogs as key candidates. In immunology, treatments based on cytokines still maintain their importance for the control of autoimmune disease, with global organizations such as the GACD sponsoring the study of interleukin inhibitors for low resource settings. In the area of cardiovascular, there is use of peptide hormones like nesiritide for acute heart failure however, the use is limited due to competition from well accepted oral therapies.
To learn more about this report, Download Free Sample
North America is expected to maintain a dominant position in the global Generic Sterile Injectables Market Outlook during the forecast period. This growth is driven by the introduction of new generic injectable products in the region. For example, in February 2022, Dr. Reddy's Laboratories Ltd launched a vasopressin injection in the U.S. market. This product, which is used to manage conditions such as frequent urination, increased thirst, and dehydration associated with diabetes, is a generic version of Par Pharmaceuticals' Vasostrict. Par Pharmaceuticals, a key player in the injectable market, specializes in developing generic and branded injectable pharmaceutical products.
Europe is poised for significant growth in the Generic Sterile Injectables Market Analysis, largely due to the increasing prevalence of chronic diseases such as cardiovascular conditions, Alzheimer's disease, and hypertension. For example, as of March 2024, NHS England reported approximately 482,000 people of all ages in England with a recorded dementia diagnosis. Meanwhile, the Dementia Statistics Hub notes there are an estimated 944,000 people living with dementia across the entire UK in 2024 Given that Alzheimer’s disease accounts for around 60% of all dementia cases, this large and growing patient population underlines the urgent need for reliable, affordable injectable therapies—such as sterile injectables used for palliative care, hydration, and management of behavioral symptoms—in both hospital and long-term care settings.
The Asia Pacific region is projected to experience substantial growth in the global generic sterile injectables market, driven by the increasing healthcare needs of the rapidly expanding populations. Countries like India and China, which have large healthcare infrastructures, are seeing rising demand for injectable medicines due to the growing incidence of chronic diseases and expanding healthcare access. Additionally, with the rising number of healthcare investments, especially in countries like India, the market for sterile injectables is expanding rapidly.
The U.S. holds the largest share within North America Generic Sterile Injectables Market Value due to a robust demand for affordable healthcare solutions and the continuous introduction of generic injectables. In February 2022, Dr. Reddy’s Laboratories launched vasopressin injection in the U.S. market—a generic alternative to Par Pharmaceuticals’ Vasostrict. This launch reflects a broader trend of increasing FDA approvals for generic injectable products. The U.S. also benefits from the presence of major pharmaceutical companies, a growing elderly population with chronic illnesses, and government support for cost-saving generic alternatives.
India represents a key growth Generic Sterile Injectables Market Trends in the Asia Pacific region. The country is one of the world's largest producers of generic drugs and sterile injectables, with significant manufacturing capacity and a skilled pharmaceutical workforce. Indian pharmaceutical companies are increasingly supplying both domestic and international markets. Government initiatives like the Production-Linked Incentive (PLI) Scheme further support local manufacturing. Additionally, rising healthcare awareness and demand for cost-effective chronic disease treatments are fueling domestic consumption of generic injectables.
China is experiencing steady growth in the generic sterile injectables market, backed by a focus on expanding healthcare infrastructure and enhancing local pharmaceutical manufacturing capabilities. The Chinese government is actively supporting the use of generics through its centralized procurement policy, which promotes price competition and broader access. The rise in chronic conditions like diabetes, cardiovascular diseases, and cancer, coupled with China’s ongoing healthcare reforms, makes the market highly favorable for generic injectables.
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 46.43 Bn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 10.5% | 2032 Value Projection: | USD 93.39 Bn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Baxter International Inc., AstraZeneca plc, Merck & Co., Inc., Pfizer Inc., Fresenius Kabi, Novartis International AG, Teva Pharmaceuticals, Hikma Pharmaceuticals, Dr. Reddy’s Laboratory, Mylan N.V., Sun Pharmaceutical Industries Ltd. |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Key players are focused on receiving approvals from the regulatory authorities and this is expected to drive growth of the global generic sterile injectables market over the forecast period. For instance, in 2025, Sandoz, a unit of Novartis AG, announced the approval from the U.S. Food and Drug Administration (FDA) for the generic version of Copaxone which is indicated for the treatment of patients with relapsing forms of multiple sclerosis.
Moreover, for instance, in 2019, Pfizer Inc. announced that the U.S. Food and Drug Administration (FDA) has approved ZIRABEV (bevacizumab-bvzr), a biosimilars to Avastin (bevacizumab), 1 for the treatment of five types of cancer: metastatic colorectal cancer; unrespectable locally advanced, recurrent or metastatic non-squamous non-small cell lung cancer (NSCLC); recurrent glioblastoma; metastatic renal cell carcinoma (RCC); and persistent, recurrent or metastatic cervical cancer.
In January 2025, Viatris Inc. was granted FDA sanction pertaining to a generic version of widely consumed oncology injectables, placing the firm in a favorable position to dominate the largely competitive oncology care market. The approval shows Vyatris’ bolstering efforts to enable access to affordable, high quality therapeutics injectables in important areas of medicine.
Major players operating in the global generic sterile injectables market include Baxter International Inc., AstraZeneca plc, Merck & Co., Inc., Pfizer Inc., Fresenius Kabi, Novartis International AG, Teva Pharmaceuticals, Hikma Pharmaceuticals, Dr. Reddy’s Laboratory, Mylan N.V. and Sun Pharmaceutical Industries Ltd.
Prescribers increasingly favor generic sterile injectables (GSIs) due to their cost-effectiveness, clinical equivalence to branded drugs, and alignment with institutional cost-containment goals. Preferences vary by care setting, treatment stage, and supply chain dynamics.
Emergency and ICU protocols prioritize generics for rapid, first-line treatment:
Generics dominate first-line therapies due to tight reimbursement policies:
Share
Share
About Author
Vipul Patil is a dynamic management consultant with 6 years of dedicated experience in the pharmaceutical industry. Known for his analytical acumen and strategic insight, Vipul has successfully partnered with pharmaceutical companies to enhance operational efficiency, cross broader expansion, and navigate the complexities of distribution in markets with high revenue potential.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients