Generic oncology sterile injectabless are biologics that have the same active ingredients as that of the branded version, with only the inactive contents of the drugs being different. Manufacturers of generic sterile injectabless have to adhere to regulations by the U.S. Food and Drug Administration (FDA) for the development of these injectabless, which are equivalent to that of branded counterparts. These generic oncology sterile injectabless are used for the treatment of various types of cancer including breast, prostate, colorectal, melanoma, lung, and bronchus. These injectabless are available at low prices and therefore, are widely preferred over branded drugs.
Furthermore, R&D for generic drugs require less capital, which increase competition to enter into generic oncology sterile injectabless market as soon as the patent for branded injectabless expire. However, the emergence of biosimilars have increased in the recent past. After Europe, increasing number of biosimilars are approved in the U.S. market. This is expected to drive growth of the generic oncology sterile injectables market growth during the forecast period.
The global generic oncology sterile injectables market size is estimated to be valued at US$ 11,496.7 million in 2019 and is expected to witness a CAGR of 11.5% over the forecast period (2019 – 2027).
Global Generic Oncology Sterile injectables Market Share (%) Analysis, By Product Type, 2019
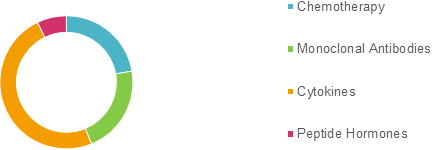
To learn more about this report, Download Free Sample
Source: Coherent Market Insights Analysis (2020)
Increasing launches of drugs is expected to drive growth of the global generic oncology sterile injectables market
Manufacturers in the U.S. are focusing on launching new products to offer low cost treatment to patients. For instance, in November, 2017, Mylan N.V. launched Clofarabine Injection (20 mg/20 mL (1 mg/mL) Single-Dose Vials) following the U.S. Food and Drug Administration approval in the U.S. market. Clofarabine is a generic version of Genzyme's Clolar, which is used for the treatment of refractory acute lymphoblastic leukemia in patients aged 1 year to 21 years.
Global Generic Oncology Sterile injectables Market – Restraints
Stringent rules and regulations for manufacturing of sterile oncology injectabless, high quality and care required during manufacturing, challenges in storage, packaging, and distribution of oncology sterile injectables are factors restraining the global generic oncology sterile injectables market growth.
Global Generic Oncology Sterile injectables Market - Regional Insights
On the basis of region, the global generic oncology sterile injectables market is segmented into North America, Latin America, Europe, Asia Pacific, Middle East, and Africa. North America holds dominant position in the global generic oncology sterile injectables market, owing to increasing launches of products in the U.S. For instance, in September 2019, Mylan N.V. launched Fulvestrant Injection, 250 mg/5 mL (50 mg/mL) per single-dose prefilled syringe, a generic version of AstraZeneca's Faslodex Injection, for the treatment of advanced breast cancer in the U.S.
Asia Pacific is the fastest growing region in the global generic oncology sterile injectables market, owing to increasing initiatives taken by key players in this region. For instance, in 2017, Alembic Pharmaceutical Limited launched manufacturing facility in India to manufacture generic oncology products for international market.
Global Generic Oncology Sterile injectables Market Value (US$ Mn) & Y-o-Y Growth (%), 2016-2027
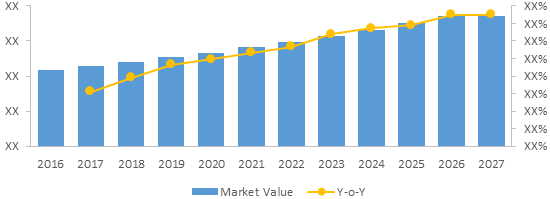
To learn more about this report, Download Free Sample
Source: Coherent Market Insights Analysis (2020)
Global Generic Oncology Sterile injectables Market - Competitive Landscape
Key players operating in the global generic oncology sterile injectables market include Eli Lilly & Company, Biocon Ltd., Baxter International Inc., Hikma Pharmaceuticals PLC, Mylan N.V., Sandoz International GmbH, Teva Pharmaceutical Industries Ltd., and Pfizer Inc.
Share
Share
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients