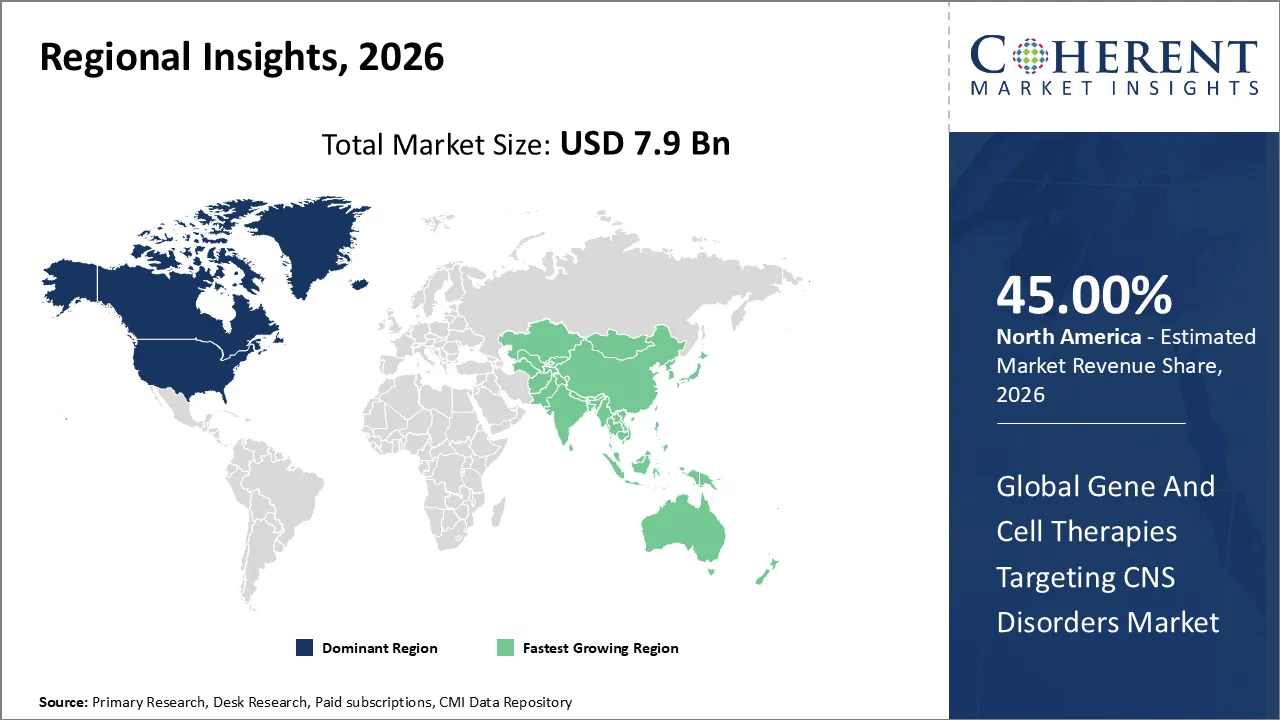
The global Gene And Cell Therapies Targeting CNS Disorders market is estimated to be valued at USD 7.9 Bn in 2026 and is expected to reach USD 24.0 Bn by 2033, growing at a compound annual growth rate (CAGR) of 16.5% from 2026 to 2033.
Increasing prevalence of neurodegenerative and neurological disorders such as Parkinson's disease, Alzheimer's disease, and Huntington's disease are the major factors driving the growth of the gene and cell therapies targeting CNS disorders market. Gene and cell therapies offer promising long-term or curative treatments for CNS disorders, which conventional therapies cannot, thereby making them highly appealing to healthcare providers and patients. Additionally, increasing developments in gene-editing technologies, viral vector delivery systems, and stem cell research are driving market growth. Growing investments by pharmaceutical and biotechnology companies, along with strong government support and favorable regulatory initiatives, further fuel the market growth of gene and cell therapy treatments for CNS disorders.
|
Current Events |
Description and its impact |
|
Regulatory Framework Evolution and Policy Changes |
|
|
AI and Digital Health Integration in CNS Treatment |
|
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
By therapy type, the gene therapy segment is estimated to contribute the highest market share of 55% in 2026, owing to its advanced clinical programs and near-term approvals. Gene therapies are increasingly preferred in CNS disorders as they target underlying genetic causes of diseases like Huntington’s and Parkinson’s, offering potential long-term or curative effects. The availability of novel delivery technologies, such as viral vectors and CRISPR-based approaches, further strengthens the adoption of gene therapy over other modalities.
For instance, in November 2025, the U.S. FDA approved Itvisma® (onasemnogene abeparvovec‑brve), a gene replacement therapy for SMA patients 2 years and older, expanding gene therapy access beyond infants.
By indication, the Parkinson’s Disease segment is predicted to occupy the largest share with 28% in 2026. It possesses the highest number of actively pursued clinical trials for CNS diseases, with Gene Therapy and Cell Therapy in advanced stages. The aim of research in Parkinson’s disease is to regain lost neurological functions, thereby decelerating disease progression, which makes it the driving force for market demand. For example, clinical research involving Gene Therapy and Stem Cell Transplant for Parkinson's will significantly influence its therapy in the future.
For instance, in September 2025, Bayer started the pivotal Phase III trial of bemdaneprocel, a cell therapy for Parkinson’s disease. The trial, called exPDite‑2, aims to replace lost dopamine-producing neurons and represents one of the first late-stage cell therapy efforts in Parkinson’s.
To learn more about this report, Download Free Sample
North America is forecasted to have the highest growth in the markets for gene & cell therapies used to treat CNS disorders in 2026, with an approximate market share of 45%. These factors could be attributed to the established presence of major biotech and pharma firms, well-equipped research facilities, and favorable regulations that accelerate the development of gene & cell therapies.
For instance, in September 2025, Bayer (via BlueRock Therapeutics) initiated the pivotal Phase III trial of bemdaneprocel for Parkinson’s disease. The trial represents one of the first late-stage cell therapy programs in the region targeting CNS disorders. The therapy is designed to replace lost dopamine-producing neurons, showcasing North America’s leadership in advancing cutting-edge therapies for neurodegenerative diseases.
The Asia Pacific market is expected to be the fastest-growing due to the rise of investment in the healthcare industry, awareness of neurodegenerative disorders, and advancements in clinical research infrastructure in China, Japan, and India. Biotech ventures in the Asia Pacific region and collaborations with multinational companies have promoted innovations in gene and cell therapies.
For instance, in May 2025, Belief BioMed in China received IND approval for BBM‑P002, a Parkinson’s gene therapy, allowing the first clinical trial in the region. This development reflects the Asia Pacific market’s rapid adoption of gene and cell therapies for CNS disorders and its potential to contribute significantly to the global market growth.
The U.S. market is booming, fueled by strong biotech presence, advanced clinical trial infrastructure, and supportive regulatory frameworks that accelerate gene and cell therapy development. The region has become a hub for innovation in therapies targeting neurodegenerative diseases such as Parkinson’s, Alzheimer’s, and ALS.
For instance, In September 2025, Gene Solutions announced that it had secured a patent for its mitochondrial therapy platform targeting neurological diseases including Alzheimer’s and Parkinson’s, broadening the tools available to develop next‑generation therapeutics for CNS disorders. The patent strengthens R&D capabilities in the U.S. for therapies designed to address mitochondrial dysfunction, a key aspect of neurodegeneration.
The Chinese market is exhibiting fast growth owing to an increase in healthcare expenditures, favorable government regulations, and local knowledge and expertise in biotechnology. Improvements in regulations and participation in international clinical trials are making it a major player in CNS-targeted gene and cell therapy.
For instance, in August 2025, SineuGene Therapeutics announced that the NMPA cleared the IND application for SNUG01, a novel gene therapy for amyotrophic lateral sclerosis (ALS), enabling a Phase I/IIa multi‑regional clinical trial in both China and the U.S. The clearance of SNUG01’s IND marks progress in developing CNS‑focused gene therapies that span major neurodegenerative disorders.
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2025 | Market Size in 2026: | USD 7.9 Bn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2026 To 2033 |
| Forecast Period 2026 to 2033 CAGR: | 16.5% | 2033 Value Projection: | USD 24.0 Bn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Novartis, BrainStorm Cell Therapeutics, Corestem, Q Therapeutics, Helixmith, Rapa Therapeutics, Neuroplast, StemCyte, Ferrer Internacional, Neuralstem, Stemedica Cell Technologies, Libella Gene Therapeutics, Sangamo Therapeutics, Hoffmann-La Roche, LSio Gene Therapies, Eli Lilly and Company, NeuroGeneration, Brain Neurotherapy Bio (AskBio), and UniQure Biopharma. |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Increasing incidence of CNS disorders such as Parkinson's disease, Alzheimer's disease, and ALS is one of the major factors contributing to market growth. Many patients are affected by these progressive, currently incurable disorders due to an aging population worldwide. This creates strong demand for advanced therapies that can modify disease progression rather than manage symptoms, driving investments and development in gene and cell therapy solutions.
The market holds a huge potential for expansion on the heels of the emerging innovations in the domain of gene and cell therapies, which encompass viral vector-based gene therapies, CRISPR-based gene editing approaches, and the use of neural progenitor cells obtained from stem cells. The innovations that can ensure the safety and efficacy of CNS-targeted therapies are bound to present the companies with opportunities to tap the unmet medical needs in the market.
The market for gene and cell therapies targeting CNS disorders is rapidly advancing, driven by cutting-edge science and urgent need for disease-modifying treatments. Several programs are now in late-stage trials for Parkinson's, Alzheimer's, and ALS, reinforcing that the CNS disorders, long considered untreatable, are finally viable targets for therapeutic intervention. Programs such as Bayer's pivotal trial of bemdaneprocel and AskBio's AB 1005 underscore maturation in therapies designed to restore or replace damaged neuronal function.
Most therapies are utilizing sophisticated technologies, including viral vector gene delivery, stem cell-derived neural progenitors, and gene editing tools, reflecting increasing levels of complexity and precision in the various approaches. Early clinical signals indicate that not only efficacy hurdles but also safety hurdles are also being met, lending credibility to the market and encouraging further investment.
North America remains a dominant hub for research and development, benefiting from a strong biotech ecosystem, experienced clinical infrastructure, and regulatory support. At the same time, China is emerging as a key player, with several companies receiving regulatory approvals to begin first-in-country clinical trials. The combination of innovative pipelines and evolving regulatory frameworks in Asia Pacific positions the region as a future center for CNS therapy development.
The landscape is shifting away from incremental, symptom-managing treatments toward truly transformative therapies. The focus on targeted interventions for rare or complex CNS disorders demonstrates a strategic move by companies to prioritize precision medicine over broad-spectrum approaches. This market is no longer speculative; it is becoming a critical arena for demonstrating the potential of next-generation neurotherapeutics and advancing patient care in ways previously thought impossible.
Share
Share
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients