Gaucher Disease Treatment Market Size and Forecast – 2025 – 2032
The Global Gaucher Disease Treatment Market size is estimated to be valued at USD 2.1 billion in 2025 and is expected to reach USD 3.45 billion by 2032, exhibiting a compound annual growth rate (CAGR) of 6.5% from 2025 to 2032.
Global Gaucher Disease Treatment Market Overview
Gaucher disease treatment products mainly include enzyme replacement therapies (ERTs) such as imiglucerase, velaglucerase alfa, and taliglucerase alfa, which work by replacing the deficient glucocerebrosidase enzyme to break down fatty substances in the body. Another category is substrate reduction therapies (SRTs) like miglustat and eliglustat, which reduce the production of glucocerebroside, the substance that accumulates due to the enzyme deficiency.
In addition, supportive treatment products include medications to manage anemia, bone complications, and organ damage. Recently, research has also advanced toward gene therapy candidates, which aim to provide a long-term corrective solution by targeting the genetic cause of the disease.
Key Takeaways
The Enzyme Replacement Therapy segment dominates the Gaucher Disease Treatment market, accounting for a commanding share of over 60%, driven by established efficacy and wide clinical adoption.
Among regional markets, North America maintains the largest market share attributable to concentrated industry presence and progressive healthcare reimbursement regimes, while Asia Pacific presents the fastest growth prospects, driven by increased diagnosis rates and expanding healthcare infrastructure.
Gaucher Disease Treatment Market Segmentation Analysis
To learn more about this report, Download Free Sample
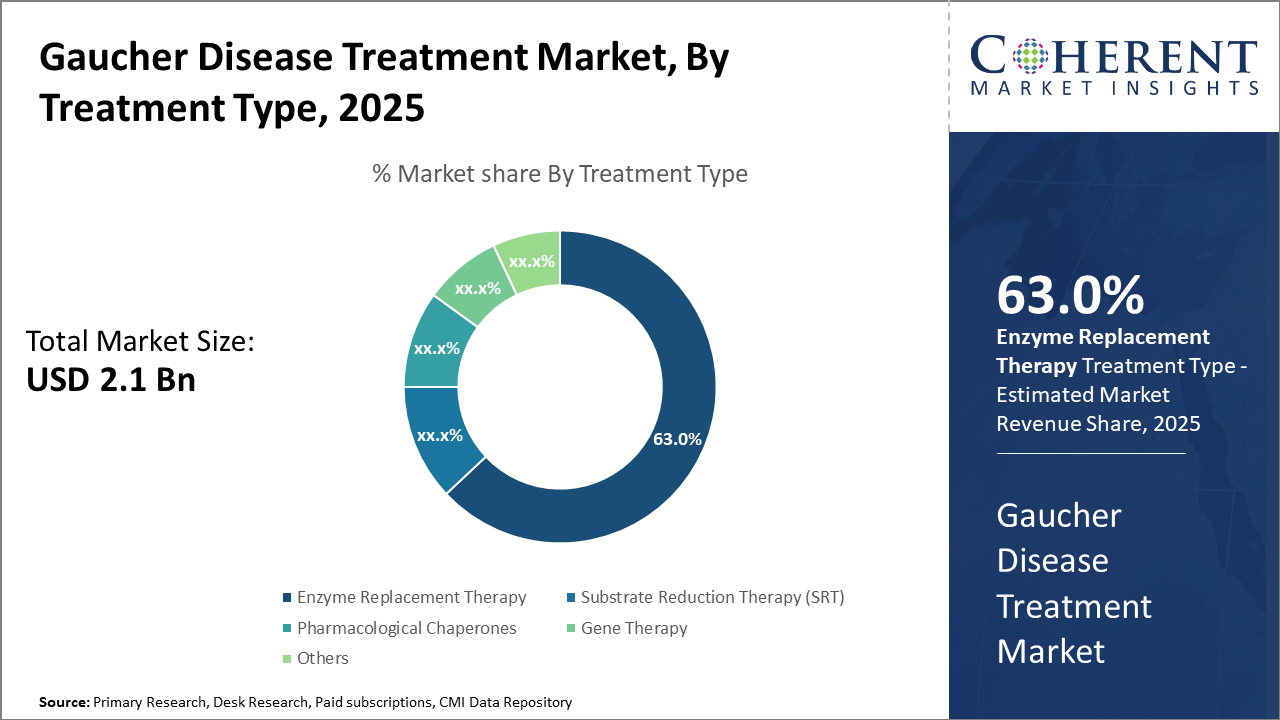
Gaucher Disease Treatment Market Insights, By Treatment Type
In terms of Treatment Type, the Enzyme Replacement Therapy dominates the market share, capturing approximately 63% due to its historically proven efficacy and wide physician familiarity. ERT remains the first-line therapy globally, supported by a steady stream of clinical data validating its impact on symptom management and organ size reduction. Substrate Reduction Therapy represents the fastest-growing segment, driven by patient preference for oral administration options and advances in molecular compounds that improve tolerability and efficacy.
Gaucher Disease Treatment Market Insights, By Patient Age Group
Adult patients hold the largest market share as they represent the majority needing ongoing treatment; comprehensive registry data indicate adults account for over 55% of diagnosed cases worldwide. Pediatric patients, however, are the fastest-growing subsegment, bolstered by enhanced newborn screening programs and earlier intervention protocols improving long-term outcomes significantly. Geriatric patients’ segment remains smaller but important, focusing on late-onset disease forms or long-term treatment management.
Gaucher Disease Treatment Market Insights, By Route of Administration
Intravenous administration dominates the market owing to the traditional reliance on enzyme replacement therapies delivered through IV infusions in clinical settings. This method ensures direct bioavailability but poses challenges related to patient convenience and adherence. Oral administration is witnessing the fastest growth trajectory, propelled by the emergence of substrate reduction therapies and pharmacological chaperones available in pill form, enhancing patient compliance and broadening market scope.
Gaucher Disease Treatment Market Trends
Market trend in the Gaucher Disease Treatment arena points to increasing adoption of gene therapy techniques, with FDA approvals of novel gene-based treatments in 2024 and 2025 underscoring this shift.
This approach promises long-term disease modification compared to lifelong enzyme replacement. Additionally, substrate reduction therapy oral formulations are gaining favor due to ease of administration, shown by a 20% increase in market revenue share in North America during 2025.
Emerging markets are witnessing growth prompted by governmental rare disease initiatives, translating into increased diagnosis and treatment uptake. For instance, India's national health programs intensified Gaucher awareness campaigns, boosting identification rates by 30% in 2024.
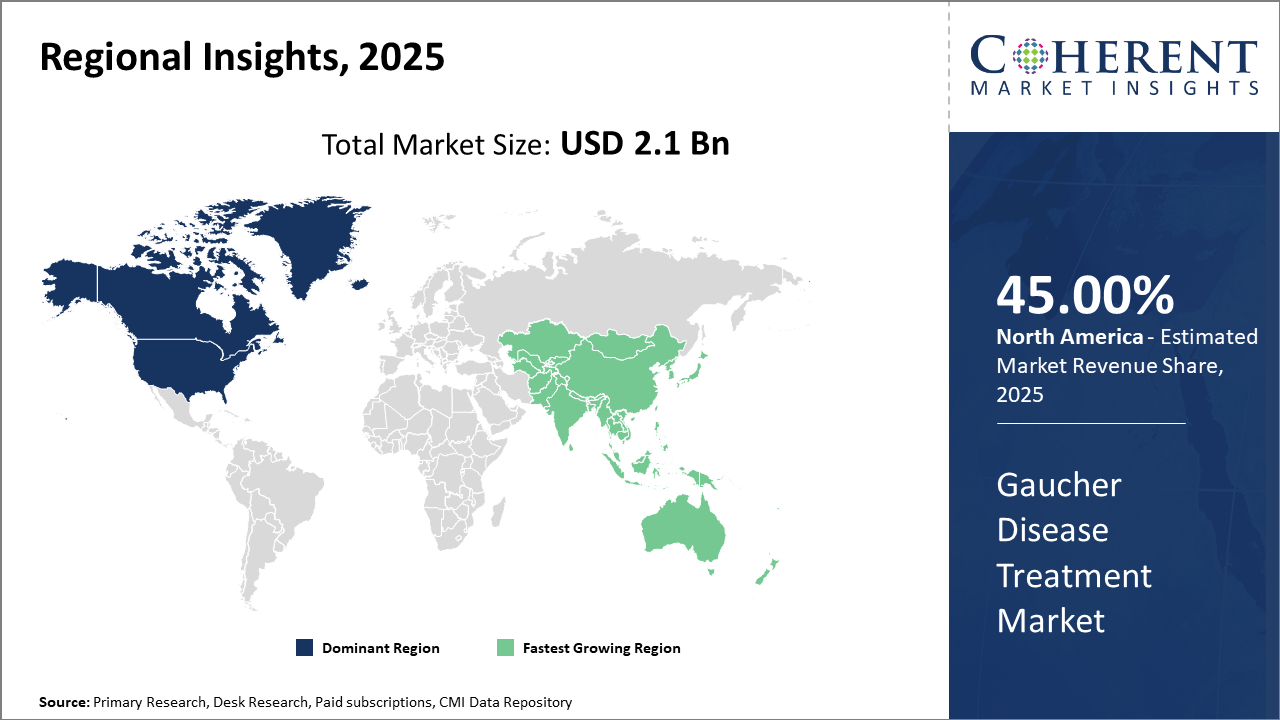
Gaucher Disease Treatment Market Insights, By Geography
To learn more about this report, Download Free Sample
North America Gaucher Disease Treatment Market Analysis and Trends
In North America, the Gaucher Disease Treatment market is led dominantly by the U.S., which contributes over 40% to the regional market share. Robust healthcare infrastructure, high per capita healthcare expenditure, and strong presence of market-leading companies facilitate this dominance. The enactment of favorable reimbursement pathways and accelerated regulatory approvals further cement North America’s leadership in industry trends and market revenue.
Asia Pacific Gaucher Disease Treatment Market Analysis and Trends
Meanwhile, the Asia Pacific exhibits the fastest growth with a CAGR exceeding 8% between 2025 and 2032. Expanding healthcare access, rising genetic disorder screening initiatives, and the growing presence of market companies investing in regions like China and India drive this trend. Government incentives to rare disease drug adoption and partnerships with international players entrench the region’s position as a burgeoning growth hub.
Gaucher Disease Treatment Market Outlook for Key Countries
USA Gaucher Disease Treatment Market Analysis and Trends
The U.S. continues to be a linchpin in the Gaucher Disease Treatment, propelled by high healthcare spending and extensive research investment. In 2025, the U.S. market revenue accounted for nearly half of the global industry size, supported by leading players like Takeda and Pfizer. FDA approval of multiple innovative therapies over recent years has enhanced treatment options, while patient advocacy groups have significantly increased disease awareness, leading to higher diagnosis rates and market demand. Additionally, strategic alliances and acquisition activities in 2024-25 have bolstered pipelines and diversified treatment offerings across enzyme replacement and gene therapies.
Germany Gaucher Disease Treatment Market Analysis and Trends
Germany’s market exemplifies strong regional performance within Europe, driven by comprehensive healthcare coverage and early adoption of advanced treatments. The country’s rare diseases framework enables swift reimbursement approvals, supporting market companies like Sanofi and Sobi in expanding their footprint. Investment in precision medicine and genetic screening has elevated patient identification, with data showing a 25% increase in treated patient base during 2024. Ongoing European Union funding initiatives further facilitate research and access, strengthening Germany’s contribution to overall European market growth and revenue.
Analyst Opinion
The expansion of enzyme replacement therapy (ERT) capacity remains a critical supply-side indicator fueling market growth. In 2024, production capabilities widened, with manufacturing expansions in Europe supporting over 30% increased throughput compared to 2023, directly boosting market availability and revenue.
On the demand side, pricing strategies have notably shifted, with several treatment providers revising cost structures to enhance affordability. For instance, innovative pricing models introduced in the US in 2025 resulted in a 15% decline in out-of-pocket expenses, broadening patient access and increasing market penetration.
Micro-indicators reveal an uptick in diagnosis rates due to the integration of next-generation sequencing technologies in clinical practice. The adoption of advanced diagnostic tools led to a 22% rise in early identification of Gaucher disease in Asia Pacific in 2024, heightening the demand for early intervention therapies.
Nano-scale factors include increasing patient-centric treatment customization, which has gained momentum in 2025 as pharmaceutical companies leveraged real-world patient data to tailor dosing regimens, improving treatment adherence and clinical outcomes. Data from clinical trials in 2025 underscore a 12% enhancement in patient compliance due to these strategies.
Market Scope
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2025 | Market Size in 2025: | USD 2.1 billion |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 6.5% | 2032 Value Projection: | USD 3.45 billion |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: | Takeda Pharmaceutical Company Limited Pfizer Inc. Sanofi S.A. Shire Plc Protalix BioTherapeutics Inc. Sobi (Swedish Orphan Biovitrum AB) Amicus Therapeutics, Inc. Vtesse, Inc. Orchard Therapeutics plc PTC Therapeutics, Inc. Inotek Pharmaceuticals Corporation. | ||
| Growth Drivers: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Gaucher Disease Treatment Market Growth Factors
The Gaucher Disease Treatment market growth is supported by increased incidence and improved screening programs worldwide, where projects initiated by health ministries in Europe have driven diagnosis rates by over 18% since 2023. Rising investments in R&D targeting gene therapy applications underpin sustained innovation that aims to reduce long-term treatment costs and disease burden, as noted by clinical trials launched predominantly in North America during 2025. Enhanced reimbursement policies across emerging markets such as Brazil and India have also removed financial barriers for patients, culminating in a rise of treatment adoption by approximately 25% over the last two years.
Gaucher Disease Treatment Market Development
In February 2025, Spur Therapeutics announced that it had a successful end-of-Phase 2 meeting with the U.S. Food and Drug Administration (FDA) for its gene therapy candidate FLT201 for Gaucher disease. The FDA aligned with Spur’s proposed design of a single-arm Phase 3 trial to support both accelerated and full approval. The company expects to dose the first patient in Phase 3 in the second half of 2025.
In May 2025, clinical data from the Phase 1/2 GALILEO trial showed that a single infusion of FLT201 produced sustained clinical benefits lasting up to 21 months in individuals with Gaucher disease type 1. The trial demonstrated improvements in key biomarkers and clinical endpoints.
Key Players
Leading Companies of the Market
Takeda Pharmaceutical Company Limited
Pfizer Inc.
Sanofi S.A.
Shire Plc
Protalix BioTherapeutics Inc.
Sobi (Swedish Orphan Biovitrum AB)
Amicus Therapeutics, Inc.
Vtesse, Inc.
Orchard Therapeutics plc
PTC Therapeutics, Inc.
Inotek Pharmaceuticals Corporation
This methodology overview emphasizes ongoing M&A activities enhancing product pipelines; for example, Takeda’s acquisition of a biotech firm specializing in gene therapies expanded its Gaucher portfolio, resulting in a 10% increase in market share in North America. Additionally, strategic partnerships between Pfizer and emerging biotech companies accelerated the development of next-generation substrate reduction therapies, improving time-to-market by nearly 9 months.
Gaucher Disease Treatment Market Future Outlook
The treatment landscape for Gaucher disease is moving toward therapies that address currently unmet needs, especially the neurological manifestations of certain disease types. Gene therapies are being developed with the potential to provide long-lasting or even one-time treatments by correcting the underlying gene defect. Small-molecule therapies able to penetrate the brain are under study in late-stage clinical trials. Improved diagnostic uptake and better awareness are expected to bring more patients into treatment earlier, potentially reducing complications. Regional expansion and regulatory incentives for rare disease treatments are likely to enhance access globally.
Gaucher Disease Treatment Market Historical Analysis
Gaucher disease has been known since the late 19th century, with its biochemical cause (deficiency of the glucocerebrosidase enzyme) established in the mid-20th century. Early treatments included splenectomy and bone marrow transplantation, efforts that attempted to manage symptoms rather than address the root cause. Over time, enzyme replacement therapies (ERTs) became available, offering recombinant versions of the missing enzyme that dramatically improved outcomes in visceral, hematologic, and bone disease. Substrate reduction therapies (SRTs) emerged later, offering an oral alternative, especially for patients who could not receive or tolerate ERT. Diagnostic tools have also evolved, shifting from invasive biopsies to enzyme activity assays and genetic testing, enabling more accurate and earlier diagnosis.
Sources
Primary Research Interviews:
Clinical Geneticist
Hematologists
Rare Disease Researchers
Databases:
PubMed
ClinicalTrials.gov
NCBI Bookshelf
Magazines:
Nature Reviews Drug Discovery
Orphanet Journal of Rare Diseases
Rare Disease Report
Journals:
Journal of Inherited Metabolic Disease
Molecular Genetics and Metabolism
Current Drug Targets
Frontiers in Genetics
Newspapers:
The Guardian (Health)
The New York Times (Science)
The Times of India (Health)
Associations:
National Gaucher Foundation
European Working Group on Gaucher Disease (EWGGD)
International Society for Rare Diseases
Share
Share
About Author
Vipul Patil is a dynamic management consultant with 6 years of dedicated experience in the pharmaceutical industry. Known for his analytical acumen and strategic insight, Vipul has successfully partnered with pharmaceutical companies to enhance operational efficiency, cross broader expansion, and navigate the complexities of distribution in markets with high revenue potential.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients