Gastrointestinal Stents Market Size and Forecast – 2025 – 2032
The Global Gastrointestinal Stents Market size is estimated to be valued at USD 1.85 billion in 2025 and is expected to reach USD 3.27 billion by 2032, exhibiting a compound annual growth rate (CAGR) of 8.3% from 2025 to 2032.
Global Gastrointestinal Stents Market Overview
Gastrointestinal stents are minimally invasive medical devices used to maintain patency in obstructed sections of the digestive tract, including the esophagus, biliary ducts, colon, and duodenum. Product types include self-expanding metal stents (SEMS), plastic stents, and biodegradable variants. SEMS are commonly made of nitinol or stainless steel and designed for long-term placement, while plastic stents are typically used for temporary relief of benign strictures. Biodegradable stents represent the latest advancement, eliminating the need for removal and reducing complications.
Key Takeaways
The Gastrointestinal Stents Market product segment is predominantly led by Self-Expandable Metallic Stents (SEMS), accounting for 62% of the share, driven by technological maturity and clinician preference.
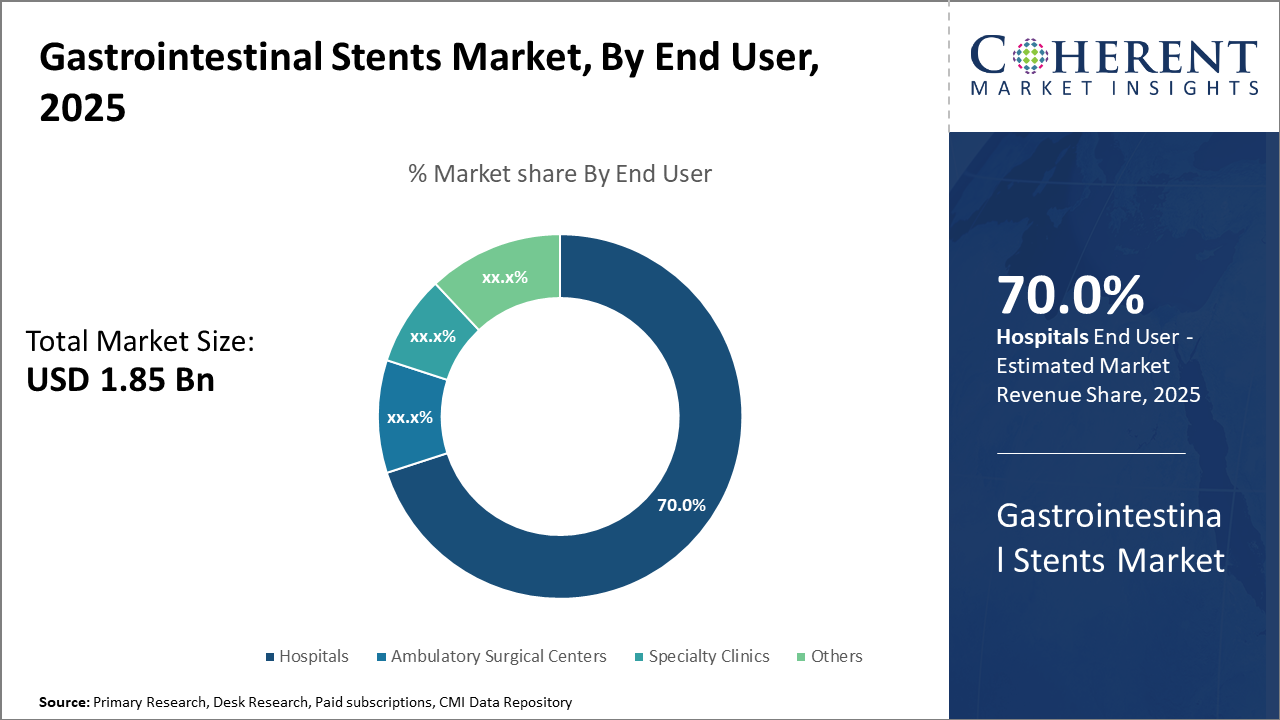
Among applications, benign strictures present a rapidly growing opportunity owing to rising chronic conditions and expanding approvals for biodegradable stent use. Hospitals remain the main end users, commanding 70% market share, supported by increasing procedural volumes and facility upgrades.
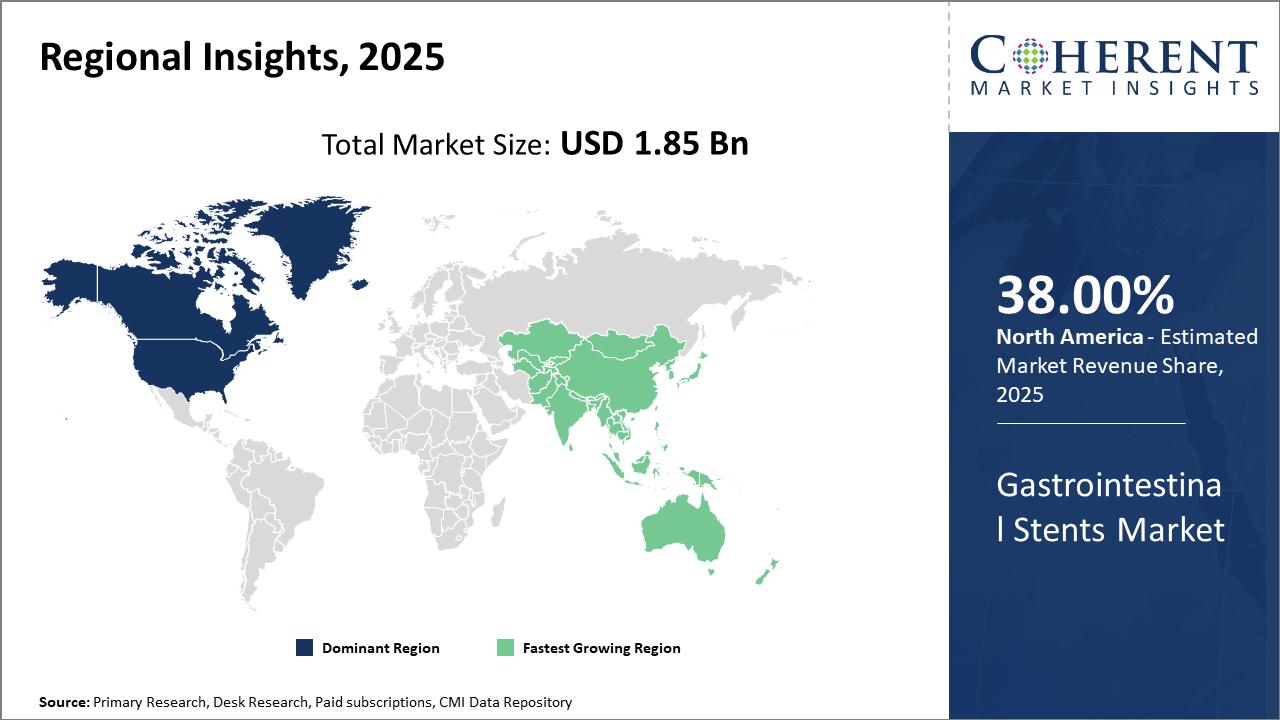
Geographically, North America dominates the Gastrointestinal Stents Market share with approximately 38% industry revenue, underpinned by strong healthcare infrastructure and reimbursement frameworks.
Asia Pacific emerges as the fastest-growing region with a CAGR exceeding 10%, driven by favorable government policies, expanding healthcare access, and local manufacturing initiatives.
Gastrointestinal Stents Market Segmentation Analysis
To learn more about this report, Download Free Sample
Gastrointestinal Stents Market Insights, By Product Type
Self-expandable metallic Stents dominate the market share as they offer superior flexibility and radial force, making them preferable for both malignant and benign gastrointestinal obstructions. Their wide clinical acceptance is a key driver, accounting for 62% of the market revenue. Biodegradable stents represent the fastest-growing subsegment, gaining traction due to their transient nature, reducing removal-related complications. Plastic stents, though economical and widely used historically, are gradually losing market share due to limitations related to biofilm formation. Balloon-Expandable Stents find niche applications in precise anatomical locations requiring controlled deployment.
Gastrointestinal Stents Market Insights, By Application
Malignant Strictures hold the dominant share, driven by the high incidence of cancers such as esophageal and colorectal cancer requiring palliative stenting. This segment benefits from continuous innovations in drug-eluting variants to combat tumor ingrowth. Benign Strictures are the fastest-growing application, escalating due to rising chronic inflammatory diseases and post-surgical complications that necessitate long-term stenting solutions. Fistulas and Perforations account for smaller but clinically critical subsegments, requiring customized stent designs for sealing and healing.
Gastrointestinal Stents Market Insights, By End User
Hospitals dominate this segment with a commanding 70% market share driven by high procedural volumes, advanced therapeutic endoscopy units, and strong purchasing power. Large teaching hospitals and cancer centers contribute substantially to this share through early adoption of innovative stent technologies. Ambulatory Surgical Centers represent the fastest-growing subsegment, benefiting from the shift towards outpatient minimally invasive procedures and cost-effective care models. Specialty Clinics focus on niche applications such as pediatric gastroenterology and offer specialized stent placements.
Gastrointestinal Stents Market Trends
Market trend analysis reveals an accelerating transition towards advanced stent technologies such as drug-eluting and biodegradable stents, which reduce post-procedural complications and improve patient compliance.
The increasing integration of AI and IoT devices in gastrointestinal stents offers real-time monitoring and personalized therapeutic strategies, as demonstrated by pilot programs in North America during 2024.
Furthermore, affordability and policy-driven accessibility in the Asia Pacific have propelled this region to the forefront of market expansion, with significant contributions from emerging domestic manufacturers.
Gastrointestinal Stents Market Insights, By Geography
To learn more about this report, Download Free Sample
North America Gastrointestinal Stents Market Analysis and Trends
In North America, the dominance in the Gastrointestinal Stents Market is underpinned by advanced healthcare infrastructure, high prevalence of gastrointestinal diseases, and favorable reimbursement scenarios. The U.S. accounts for over 30% of the global market share, supported by leading companies like Boston Scientific and Medtronic, driving continuous innovation. Strong burn-in of endoscopic procedures and research collaborations fosters sustained business growth.
Asia Pacific Gastrointestinal Stents Market Analysis and Trends
Meanwhile, the Asia Pacific exhibits the fastest growth, with a CAGR exceeding 10%, catalyzed by expanding healthcare spending, government initiatives to improve healthcare access, and rapid urbanization. China and India contribute majorly due to the rising patient pool and increasing hospital networks adopting gastrointestinal stenting technologies, coupled with growing domestic manufacturing capabilities.
Gastrointestinal Stents Market Outlook for Key Countries
USA Gastrointestinal Stents Market Analysis and Trends
The USA's Gastrointestinal Stents Market is characterized by high investments in research and development, especially in smart stents and drug-eluting technologies. National Cancer Institute reports indicate an increasing incidence of gastrointestinal cancers, maintaining steady demand. The presence of several top market companies and advanced healthcare reimbursement systems accelerates the adoption of innovative stent technologies. Furthermore, clinical trials conducted in leading U.S. institutions help refine device efficacy, reinforcing the country's influential role in market revenue generation and technological leadership.
Japan Gastrointestinal Stents Market Analysis and Trends
Japan’s market benefits from an aging population with increased gastrointestinal disorder prevalence, encouraging adoption of minimally invasive therapeutic options. The government’s healthcare policies promote innovation and reimbursement for biodegradable stents, elevating regional business growth. Japanese companies and local subsidiaries of international players collaborate closely to develop next-gen stents compatible with endoscopic technology standards in the APAC region. Clinical data from 2025 highlight Japan’s leading role in biodegradable stent clinical application and commercialization initiatives.
Analyst Opinion
The rising adoption of self-expandable metallic stents (SEMS) remains a pivotal supply-side indicator driving market expansion. Recent hospital procurement data from 2024 revealed a 15% annual increase in SEMS utilization in therapeutic endoscopy, underscoring product reliability and clinical preference. Europe alone accounted for an approximate 40% share in SEMS placement procedures in 2024, reflecting its dominance.
Demand-side dynamics highlight the increasing use of gastrointestinal stents in palliative care for esophageal and colorectal cancers, where stent placement reduces surgical intervention rates by 23% as seen in U.S. cancer treatment centers during 2023-2024. This shift towards less invasive modalities backed by clinical guidelines adoption has amplified market revenue.
The escalating pipeline of innovative biodegradable stents showcased in trials globally, particularly in Asia Pacific countries, points toward a micro-indicator of future growth. For instance, clinical trials in Japan demonstrated a 12% faster mucosal healing rate using biodegradable stents in benign strictures in early 2025, affirming industry trend acceleration.
Pricing strategies and reimbursement policies significantly influence market scope; the U.S. Centers for Medicare & Medicaid Services (CMS) updated their reimbursement codes in 2024 to moderately increase coverage on novel stent devices, fostering increased hospital adoption and business growth. This policy adjustment impacted market share positively by approximately 5% across North America last year.
Market Scope
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2025 | Market Size in 2025: | USD 1.85 billion |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 8.3% | 2032 Value Projection: | USD 3.27 billion |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: | Boston Scientific Corporation, Cook Medical, Medtronic plc, S&G Biotech, Nitinol Devices & Components, Inc., Becton Dickinson and Company, Micro-Tech Endoscopy USA, Inc., Olympus Corporation, Nanjing Micro-Tech Medical Company Limited, Leufen Medical GmbH, Hangzhou Endovision Technology Co., Ltd. | ||
| Growth Drivers: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Gastrointestinal Stents Market Growth Factors
The increasing prevalence of gastrointestinal cancers, especially esophageal and colorectal cancers, is a primary market driver, backed by WHO 2024 statistics showing an 8% annual rise in cancer incidence globally. Enhanced early diagnosis and adoption of endoscopic palliative treatments have propelled market growth. Rising geriatric populations worldwide escalate demand for minimally invasive interventions, with the global senior population increasing by 5.5% annually, thereby expanding the target demographic for gastrointestinal stents.
Technological advancements in stent materials, notably biodegradable and drug-eluting variants, have improved safety and efficacy, capturing new patient segments—clinical trials in 2025 reported up to 30% reduction in restenosis rates with these innovations. Expanding hospital infrastructure and growing healthcare expenditure in emerging markets, especially within the Asia Pacific, have facilitated increased access to advanced gastrointestinal stenting solutions.
Gastrointestinal Stents Market Development
In June 2022, Boston Scientific completed its acquisition of M.I.Tech Co. Ltd., a Korean company, for approximately $230 million. This deal was intended to expand Boston Scientific's portfolio of interventional devices and its presence in the non-vascular stenting market by acquiring M.I.Tech's HANAROSTENT technology, a line of self-expanding nitinol stents used in the gastrointestinal and airway systems.
In February 2023, the global medtech company Olympus Corporation acquired Taewoong Medical, a South Korean manufacturer specializing in gastrointestinal stents. It is likely that the stent referred to as the "T-Stent Biliary Stent" in the prompt is a product developed by Taewoong and is part of the "Niti-S™ Biliary Stent" family.
Key Players
Leading Companies of the Market
Boston Scientific Corporation
Cook Medical
Medtronic plc
S&G Biotech
Nitinol Devices & Components, Inc.
Becton Dickinson and Company
Micro-Tech Endoscopy USA, Inc.
Olympus Corporation
Nanjing Micro-Tech Medical Company Limited
Leufen Medical GmbH
Hangzhou Endovision Technology Co., Ltd.
Several leading players have implemented strategic collaborations to expand their market footprint. For instance, Boston Scientific’s partnership with regional healthcare providers in the Asia Pacific resulted in a 20% revenue uptick in 2024, bolstered by localized production and distribution efficiencies. Meanwhile, Medtronic’s launch of innovative drug-eluting stents in 2025 exemplifies product diversification aimed at enhancing therapeutic efficacy.
Gastrointestinal Stents Market Future Outlook
The future of the Gastrointestinal Stents Market looks promising as ongoing innovation in bioresorbable and drug-eluting stents enhances patient outcomes and long-term patency. With increasing emphasis on outpatient endoscopic care and the integration of imaging-guided navigation systems, procedure safety and success rates are expected to improve substantially. Growing demand for palliative care, aging demographics, and the rising global burden of GI malignancies will drive adoption further. The next decade will likely see strong penetration in Asia-Pacific and Latin America as healthcare infrastructure expands, propelling the market toward consistent mid-single-digit growth through 2032.
Gastrointestinal Stents Market Historical Analysis
The Gastrointestinal Stents Market has evolved in tandem with advancements in minimally invasive procedures and interventional endoscopy. Historically, stent placement was reserved for palliative management of malignant obstructions, but the emergence of self-expanding metal stents (SEMS) transformed clinical practice by reducing complications and hospital stays. Through the 2010s, technological innovation in stent coatings, delivery precision, and anti-migration designs helped expand applications from esophageal and biliary use to colorectal and duodenal indications. Rising incidence of gastrointestinal cancers and improved access to interventional gastroenterology in developed markets were key historical growth drivers, while reimbursement inconsistencies and limited expertise in some regions restrained wider adoption.
Sources
Primary Research Interviews:
Gastroenterologists
Interventional Endoscopists
Surgical Oncologists
Hospital Procurement Managers
Biomedical Engineers
Databases:
PubMed
FDA Medical Device Database
GlobalData Gastroenterology Device Reports
Magazines:
Gastroenterology & Endoscopy News
Medical Device Network
OR Today
Endoscopy Today
Journals:
Gastrointestinal Endoscopy
Surgical Endoscopy
World Journal of Gastroenterology
Endoscopy International Open
Newspapers:
The Guardian (Health)
The New York Times (Medical)
The Hindu (Health)
The Times of India (Health)
Associations:
American Gastroenterological Association (AGA)
European Society of Gastrointestinal Endoscopy (ESGE)
World Gastroenterology Organization (WGO)
Share
Share
About Author
Manisha Vibhute is a consultant with over 5 years of experience in market research and consulting. With a strong understanding of market dynamics, Manisha assists clients in developing effective market access strategies. She helps medical device companies navigate pricing, reimbursement, and regulatory pathways to ensure successful product launches.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients