The functional service providers (FSP) market is estimated to be valued at USD 18.40 Bn in 2025 and is expected to reach USD 32.80 Bn by 2032, exhibiting a compound annual growth rate (CAGR) of 8.6% from 2025 to 2032.
Discover market dynamics shaping the industry: Download Free Sample
The functional service providers (FSP) Market has been witnessing significant growth over the past few years. The adoption of advanced technologies across various industries has been increasing productivity and efficiency. Moreover, the rising need to focus on the core business competencies and outsource non-core operations is driving the growth of the functional service providers (FSP) market. Many companies operating in this market offers specialized, industry-specific solutions to help clients focus on their core competencies. Going forward, the growing generation of huge amounts of data and increasing focus on gaining insights to make strategic business decisions are expected to present attractive opportunities for the players operating in the functional service providers (FSP) market during the forecast period.
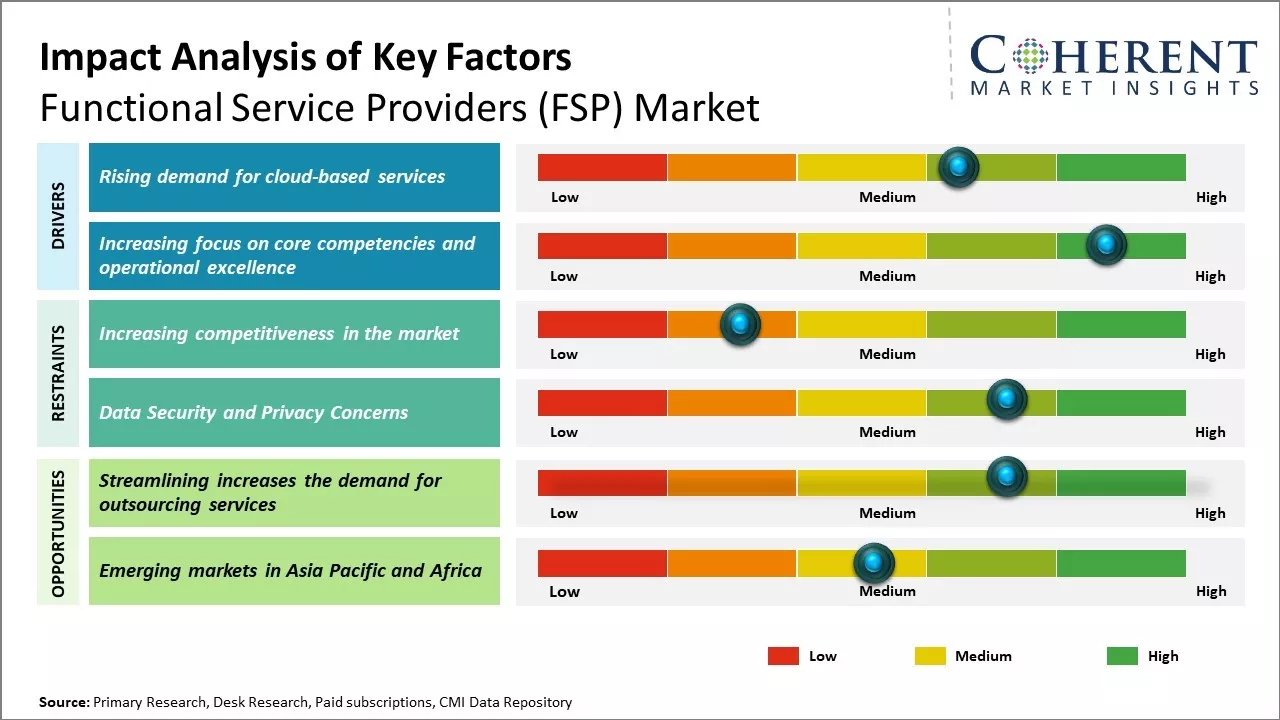
Rising demand for cloud-based services
With the rapid growth of digitization across all industries, there has been a massive increase in the volume of data being generated every minute. However, most organizations lack the in-house expertise and resources to store, manage, and analyze such huge amounts of data. This is where functional service providers (FSPs) come into the picture by offering scalable cloud-based solutions for data management. They provide data storage, analytics, and other digital services over the internet through utility-style pricing models. This on-demand availability of data-related capabilities has eliminated upfront infrastructure investments for organizations. It has allowed even small and medium enterprises to leverage advanced tools and technologies. For instance, in March 2023 Fujitsu, a Japan-based multinational information and communications technology firm, announced the launch of a new cloud-based platform that enables users to securely collect and leverage health-related data to support digital transformation in the medical industry.
Get actionable strategies to beat competition: Download Free Sample
Increasing focus on core competencies and operational excellenceAnother key driver has been the increasing focus across all businesses to shed non-core activities and concentrate organizational resources entirely on core competencies that delivers greatest competitive advantage. In the cut-throat business environment, focus on strengthening core offerings, refining business processes, reducing operational costs, and improving overall efficiency has become imperative for growth and survival. However, non-core or support functions like finance, accounting, procurement, etc. still need to be handled efficiently even as top management directs attention to key growth areas. This is driving greater openness to outsourcing such shared services to capable and specialized third-party FSPs. Businesses engaging FSPs are able to drastically streamline processes, leverage technological innovations and process expertise to handle functions at much larger scales. Advanced analytics, automation, and standardized KPI-driven processes adopted by FSPs ensure higher consistency, control and compliance. This allows internal teams to contribute more value adding tasks while gaining access to global best practices.

To learn more about this report, Download Free Sample
Market Challenges – Increasing competitiveness in the marketThe functional service providers (FSP) market faces several challenges. With increasing globalization, many organizations are shifting to shared services and outsourcing non-core functions to specialized providers. This has increased competition for FSPs as clients can easily switch between multiple options. Additionally, with rising customer expectations, FSPs need to continually invest in new technologies and digital capabilities to offer enhanced services. They also need to skill and re-skill employees to keep up with changing market needs.
Market Opportunities – Streamlining increases the demand for outsourcing services
As more companies focus on streamlining costs and improving efficiencies, the demand for functions like data management, medical writing, and clinical trial design & monitoring is growing. FSPs can expand their service offerings and target new industry segments. With digital disruption, there is a chance to provide innovative technology-led solutions and automated services, thus creating lucrative growth opportunities for the market over the projected period.
Discover high revenue pocket segments and roadmap to it: Download Free Sample
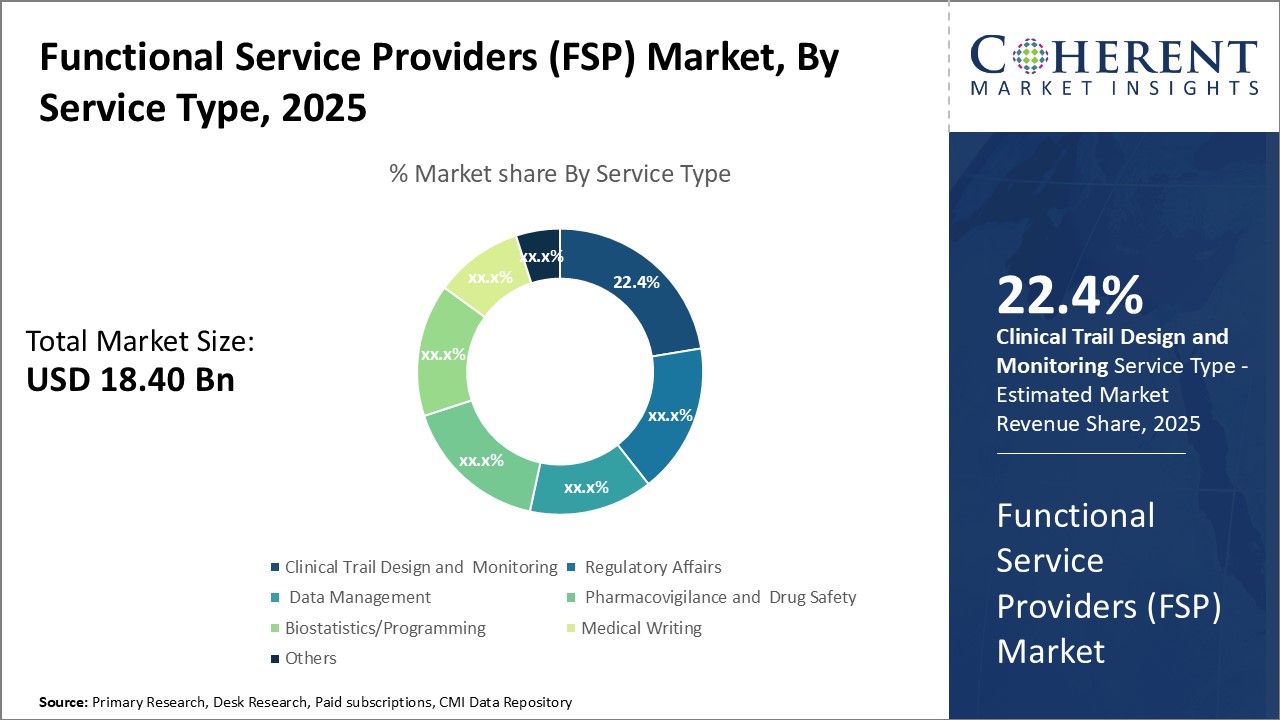
Insights, By Service Type: Increasing focus on quality in clinical research drives clinical trial design and monitoring segment growthThe service type segment includes clinical trial design and monitoring, regulatory affairs, data management, pharmacovigilance and drug safety, biostatistics/programming, medical writing, and others. The clinical trial design and monitoring segment is estimated to hold 22.4% share of the market in 2025. As clinical trials have become more complex in design and global in nature, sponsors are recognizing the need to partner with specialized Clinical Research Organizations (CROs) that have expertise in various aspects of clinical trial management. CROs assisting with clinical trial design and monitoring help ensure protocol adherence, protect patient safety, ensure data integrity and allow sponsors to concentrate on their core competencies. As regulatory requirements around clinical trials also continue to expand, the need for experienced outsourcing partners has grown. CROs provide expertise around new guidelines and regulations to help reduce sponsors' compliance risks. Their monitoring of trial sites helps identify issues early to minimize delays. As clinical research continues its transition towards a more decentralized and technologies-driven model, CROs also help sponsors navigate this change through innovative trial design and virtual/remote monitoring capabilities. This focus on quality and specialized expertise through outsourcing is a major driving factor for the clinical trial design and monitoring segment.
Insights, By Stage: Rise in the number of clinical studies is bolstering the growth of clinical development segment
The stage segment includes clinical development and post approval. The clinical development subsegment is estimated to have 70.3% of the market share in 2025. However, the post-approval segment is growing rapidly owing to the need for optimized evidence generation plans. As regulatory requirements expand post-approval, sponsors require strategic guidance and services around additional clinical trials, patient registry programs and real-world evidence studies. These efforts aim to expand approved indications, evaluate long-term safety and efficacy, and provide patients, payers and providers with robust evidence on treatment benefits. CROs with post-approval experience help sponsors navigate regulatory complexities and design optimal evidence generation strategies tailored to their products' evolving profiles. They also assist with conducting various post-marketing studies and generating publications/communications around resulting data. By outsourcing these specialized services, sponsors can focus on their commercialization efforts with the confidence that additional evidence requirements are well-managed. This need to continually optimize evidence generation drives demand in the growing post-approval segment.
Insights, By Therapeutic Area: Growing research & development drives oncology segment growth
The therapeutic area segment includes oncology, cardiovascular, infectious diseases, central nervous system (CNS), respiratory, immunology, gastrointestinal, and others. The Oncology segment accounts for the highest share of the market and is estimated to hold 27.3% of the market share in 2024. Strong partnerships between pharma/biotech sponsors and specialized oncology CROs help advance the dynamic research and development in this space. CROs provide expertise across various oncology sub-disciplines like hematology, urology, gynecology, etc. to assist with clinical trial design and regulatory strategies tailored to differing cancer types, mechanisms, and standards of care. As immuno-oncology and personalized medicine continue redefining cancer management, CROs also help sponsors design and implement innovative biomarker and companion diagnostic-led studies to accelerate new targeted therapies to patients. Their experience in complex oncology trials further aids with optimizing global site identification and management challenges for these studies. Additional services around real-world oncology data also support enhanced understanding of disease progression, response to therapies and patient quality of life over the longer term. With relentless innovation and diverse therapy options on the horizon, oncology as a therapeutic area will continue to see strong partnership-driven research - sustaining its leadership in the functional service providers (FSP) market.
Need a Different Region or Segment? Download Free Sample
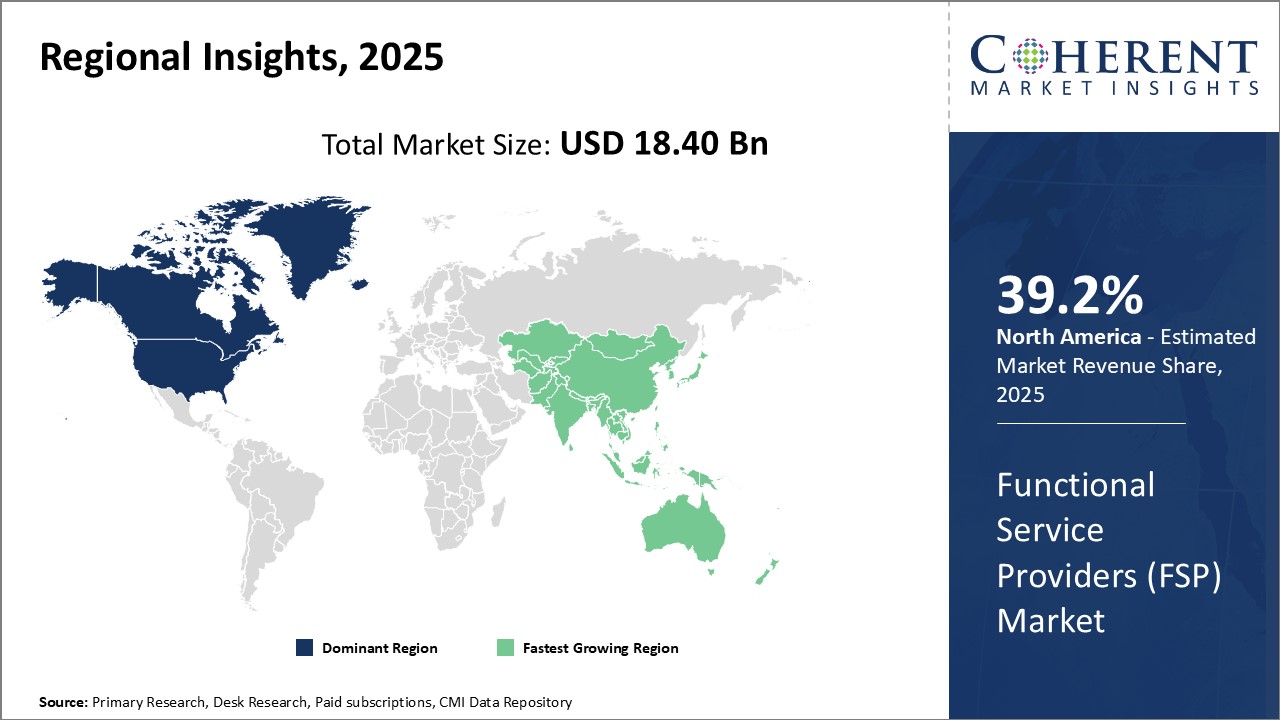
North America has established itself as the dominant region in the global functional service providers (FSP) market and is estimated to hold 39.2% of the market share in 2025. The region is home to many industry pioneers and largest FSPs owing to several favorable conditions. With a high demand for outsourcing non-core activities from large enterprises, FSPs in the U.S. and Canada have developed deep domain expertise across key industry verticals like manufacturing, healthcare, retail, etc. over the years. They leverage their expertise to deliver standardized as well as customized solutions at a scale very few global players can match. Besides client demand, the region also offers a versatile talent pool which enables FSPs to recruit and manage large workforces with required skillsets. Many local universities train graduates in relevant fields annually, ensuring talent availability. Additionally, North America has a culture of entrepreneurship which has supported the growth of homegrown as well as foreign FSPs establishing delivery centres in the region. Overall market maturity, demand predictability and talent availability have made North America the preferred destination for businesses seeking functional services.
The functional service providers (FSP) market in the Asia Pacific region is anticipated to develop at the highest CAGR. The Clinical Trial Design and Monitoring subsegment dominates the service type segment in the APAC region due to rapid growth in clinical research activities. Countries like China, Japan and South Korea are increasingly becoming global clinical research hubs due to lower costs and a large patient population. This growth is driven by expansion of the biopharma industry as well as government initiatives aimed at streamlining regulations and building research capabilities. Within the service type segment, the data management subsegment is experiencing the fastest growth in the APAC region supported by rising clinical research activity and regulatory requirements. Effective data management is critical for clinical trials given the large volumes of data generated across global study sites. The region lacks standardized processes and quality data managers compared to Western markets. Therefore, there is increased reliance on specialized functional service providers (FSPs) that offer end-to-end data management solutions.
Functional Service Providers (FSP) Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 18.40 Bn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 8.6% | 2032 Value Projection: | USD 32.80 Bn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
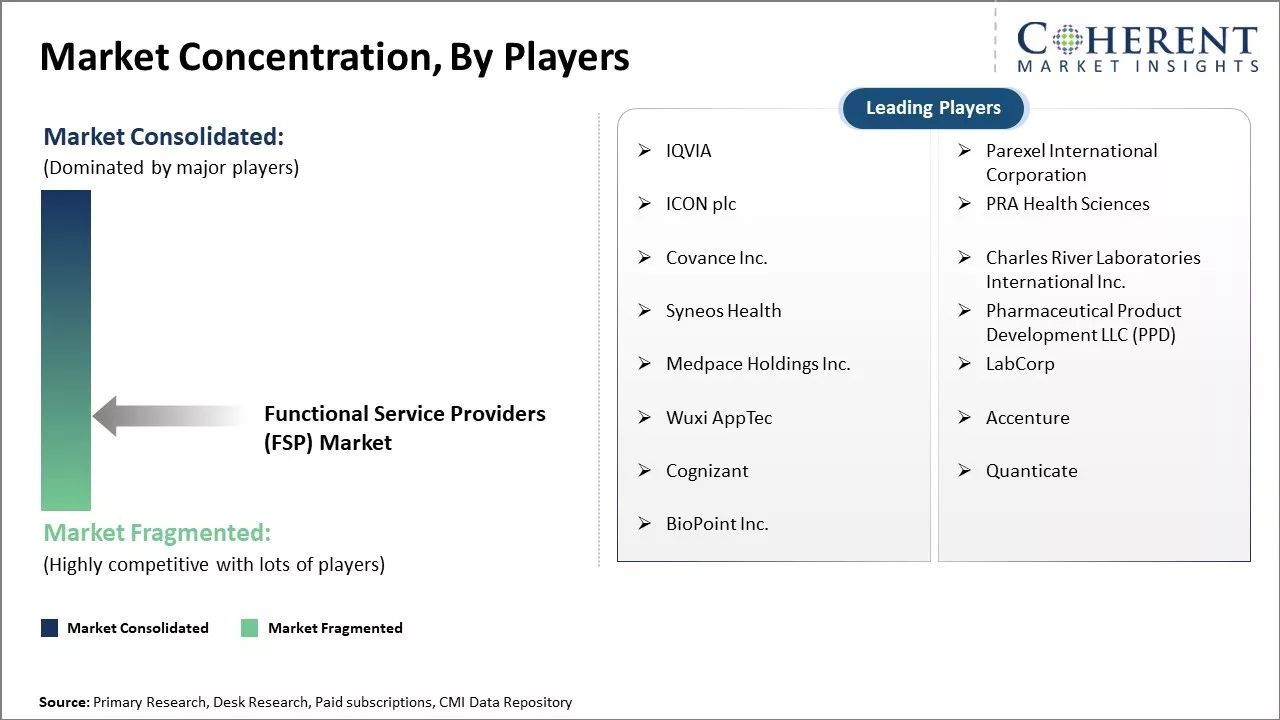
IQVIA, Parexel International Corporation, ICON plc, PRA Health Sciences, Covance Inc., Charles River Laboratories International Inc., Syneos Health, Pharmaceutical Product Development LLC (PPD), Medpace Holdings Inc., LabCorp, Wuxi AppTec, Accenture, Cognizant, Quanticate, BioPoint Inc. |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Share
Share
About Author
Ankur Rai is a Research Consultant with over 5 years of experience in handling consulting and syndicated reports across diverse sectors. He manages consulting and market research projects centered on go-to-market strategy, opportunity analysis, competitive landscape, and market size estimation and forecasting. He also advises clients on identifying and targeting absolute opportunities to penetrate untapped markets.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients