Friedreich’s ataxia is caused by genetic mutations resulting in decline in production and expression of a mitochondrial protein called frataxin, which is involved in regulating iron-sulphur cluster enzymes within cells of the body. Insufficient frataxin levels result in the reduced activity of several mitochondrial enzymes involved in the electron transport chain, which leads to impaired mitochondrial metabolism such as the accumulation of iron in mitochondria dysfunction of adenosine triphosphate synthesis, reduced ATP (Adenosine triphosphate) production, and inefficient anti-oxidant response. As a consequence of mitochondrial dysfunction, neurons progressively die and Friedreich’s ataxia patients progressively lose their ability to coordinate movement and perform daily activities such as walking, running, etc. effectively. Thus, therapeutic strategies for the management or treatment of Friedreich’s ataxia patients aim at correcting some of the downstream consequences of frataxin deficiency or at restoring sufficient frataxin levels in the cell.
However, an adequate cure for Friedreich’s ataxia is not yet available for patients. Some of the pharmacology therapy approaches used for the management or treatment of Friedreich’s ataxia patients include usage of drugs such as angiotensin-converting enzyme (ACE) inhibitors, beta blockers, diuretics, para-benzoquinone, immunomodulators, skeletal muscle relaxants, anti-epileptic drugs, nuclear factor-erythroid factor 2-related factor 2 (Nrf2) inducer, histone deacetylase (HDAC) inhibitors, deuterated polyunsaturated fatty acids, trans-activators of transcription (TAT)-frataxin, iron chelators, etc. Future therapeutics of Friedreich’s ataxia involve advanced gene therapy to add a correct copy of the gene responsible for the production of frataxin protein in the body.
The global Friedreich’s ataxia market is estimated to be valued at US$ 777.2 million in 2022 and is expected to exhibit a CAGR of 13.0% over the forecast period (2022-2030).
Figure 1. Global Friedreich’s Ataxia Market Share (%), By Region, 2022
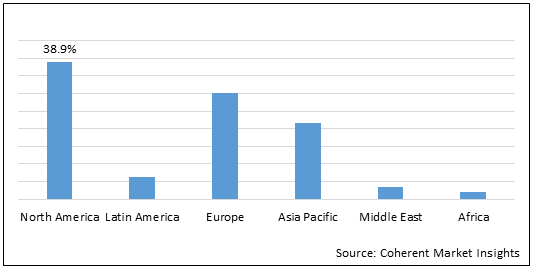
To learn more about this report, Download Free Sample
High burden of Friedreich’s ataxia is expected to drive growth of the global Friedreich’s ataxia market over the forecast period
For instance, according to data published by the Ataxia UK, a charity registered in Scotland and England & Wales in November 2020, it was estimated that the incidence of Friedreich’s ataxia based on carrier frequency of 1 in 85 was 1:29,000 in Europe in 2020.
Friedreich’s Ataxia Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2021 | Market Size in 2022: | US$ 777.2 Mn |
| Historical Data for: | 2017 to 2020 | Forecast Period: | 2022 to 2030 |
| Forecast Period 2022 to 2030 CAGR: | 13.0% | 2030 Value Projection: | US$ 2,062.9 Mn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Reata Pharmaceuticals, Inc., Retrotope Inc., Minoryx, PTC Therapeutics, Design Therapeutics, Inc., Larimar Therapeutics, Inc., Jupiter Neurosciences, Inc., Lexeo Therapeutics, Zydus Lifesciences Ltd., Cipla Limited, GlaxoSmithKline Plc., Aurobindo Pharma Ltd., Sun Pharmaceutical Industries Ltd., Torrent Pharmaceuticals Ltd., and Intas Pharmaceuticals Ltd. |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Figure 2. Global Friedreich’s Ataxia Market Value (US$ Mn), By Route of Administration, 2022
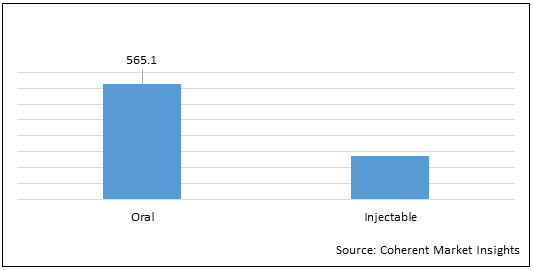
To learn more about this report, Download Free Sample
Increasing research and development activities for introduction of novel Friedreich’s ataxia therapeutics is expected to drive the market growth over the forecast period.
For instance, according to an article published in the Neurobiology of Disease journal, in January 2021, an oral drug called leriglitazone had shown benefits in treating Friedrich's ataxia in cellular and animal models. Leriglitazone is manufactured by Minoryx Therapeutics, a Spain-based clinical-stage biotechnology company, and is currently in advanced Phase 2/3 of clinical trials for the treatment of Friedrich's ataxia. Leriglitazone restores mitochondrial function which is generally hampered due to the absence of mitochondrial protein frataxin (FXN) in Friedreich's ataxia disease.
Furthermore, in February 2022, Design Therapeutics, Inc., a U.S.-based biotechnology company, announced that it is currently conducting Phase 1 clinical trial of its investigational drug DT-216, an intravenous (IV) injection for the treatment of Friedreich's Ataxia and this clinical trial study is expected to be completed by December 2022.
Key players operating in market are focusing on obtaining regulatory approval of their Friedreich’s ataxia drugs, which is expected to drive growth of the global Friedreich’s ataxia market over the forecast period.
In March 2022, Stealth BioTherapeutics Inc., a Cayman Islands-based biotechnology company, announced that it received Orphan Drug Designation from the U.S. Food and Drug Administration (US FDA) Office of Orphan Products Development for its drug Elamipretide, which is indicated for the treatment of Friedreich's ataxia.
Global Friedreich’s Ataxia Market – Impact of Coronavirus (Covid-19) Pandemic
SARS-CoV-2 (Severe acute respiratory syndrome coronavirus) was first reported from Wuhan, China in December 2019, and was declared as a global pandemic by the World Health Organization (WHO), which designates this SARS-CoV-2 infection as novel Coronavirus (COVID-19). According to the Coronavirus (COVID-19), Weekly Epidemiological Update by the World Health Organization, COVID-19 has spread across the globe infecting over 519 million population leading to more than 6.3 million deaths as of May 15, 2022. The negative impact of COVID-19 on the healthcare domain is huge and it has significantly disrupted the healthcare industries’ entire supply chain, from raw materials to manufacturing and delivery. Similarly, the COVID-19 pandemic had a negative impact on the overall growth of the Friedreich’s ataxia market, owing to movement restrictions and lockdowns.
Similarly, demand and supply for Friedreich’s ataxia therapeutics was disrupted, owing to halt of clinical trials aimed at developing treatment drugs for Freidreich’s ataxia, as well as disrupted supply of Friedreich’s ataxia treatment drugs during the lockdown. Moreover, physiotherapy, rehabilitation programs, physical training, etc. for management of Friedreich’s ataxia was at halt during the lockdown.
For instance, according to an electronic survey conducted amongst Friedreich ataxia (FA) patients in Italy and published in the Cerebellum & Ataxias journal, an open access scientific journal in January 2021, it was found that the number of patients undergoing physiotherapy was reduced by 72.2% due to the COVID-19 lockdown. According to the same source, over 60% of Friedreich ataxia patients reported worsening of their health in absence of physiotherapy during COVID-19 lockdown.
Global Friedreich’s Ataxia Market - Restraint
Challenges faced during designing and conducting clinical trials for development of Freidreich's ataxia treatment are the factors that are expected to hinder growth of the global Friedreich’s ataxia market over the forecast period.
Designing clinical trials for rare neurodegenerative diseases such as Friedreich's ataxia is tricky due to the insufficient number of Friedreich's ataxia patients, heterogeneous nature of the disease with a slow and variable progression rate, which makes it difficult to identify reliable outcome measures to monitor the progression of Friedreich's ataxia.
For instance, according to an article published by the Orphanet Journal of Rare Diseases, in August 2020, Friedreich's ataxia is a genetic, neurodegenerative movement disorder, with a typical age of onset (the age at which the symptoms of a disease first appear) between 10 and 15 years. Thus, the diagnosis of the disease takes a long time since its initial stage. The same source also estimated that the average time taken for the diagnosis of rare disorders such as Friedreich's ataxia is 5 years.
Key Players
Major players operating in the global Friedreich’s ataxia market include Reata Pharmaceuticals, Inc., Retrotope Inc., Minoryx, PTC Therapeutics, Design Therapeutics, Inc., Larimar Therapeutics, Inc., Jupiter Neurosciences, Inc., Lexeo Therapeutics, Zydus Lifesciences Ltd., Cipla Limited, GlaxoSmithKline Plc., Aurobindo Pharma Ltd., Sun Pharmaceutical Industries Ltd., Torrent Pharmaceuticals Ltd., and Intas Pharmaceuticals Ltd.
Share
Share
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients