Fractional Flow Reserve Market Size and Forecast – 2025 – 2032
The Global Fractional Flow Reserve Market size is estimated to be valued at USD 1.2 billion in 2025 and is expected to reach USD 2.05 billion by 2032, exhibiting a compound annual growth rate (CAGR) of 8.5% from 2025 to 2032.
Global Fractional Flow Reserve Market Overview
Fractional Flow Reserve (FFR) products are medical devices and adjuncts used to measure the physiological significance of coronary artery stenoses by assessing pressure differences across a lesion during induced hyperemia. Core components include specialized pressure guidewires with high-accuracy distal pressure sensors, pressure transducers/monitors, adenosine or other hyperemic agents (for some protocols), and computational/graphical display systems integrated into cath lab consoles. FFR systems come as disposable or reusable pressure guidewires with varying sensor technologies (fiber-optic, piezoelectric), user interfaces that display Pd/Pa and FFR values, and software tools for recording and reporting results.
Key Takeaways
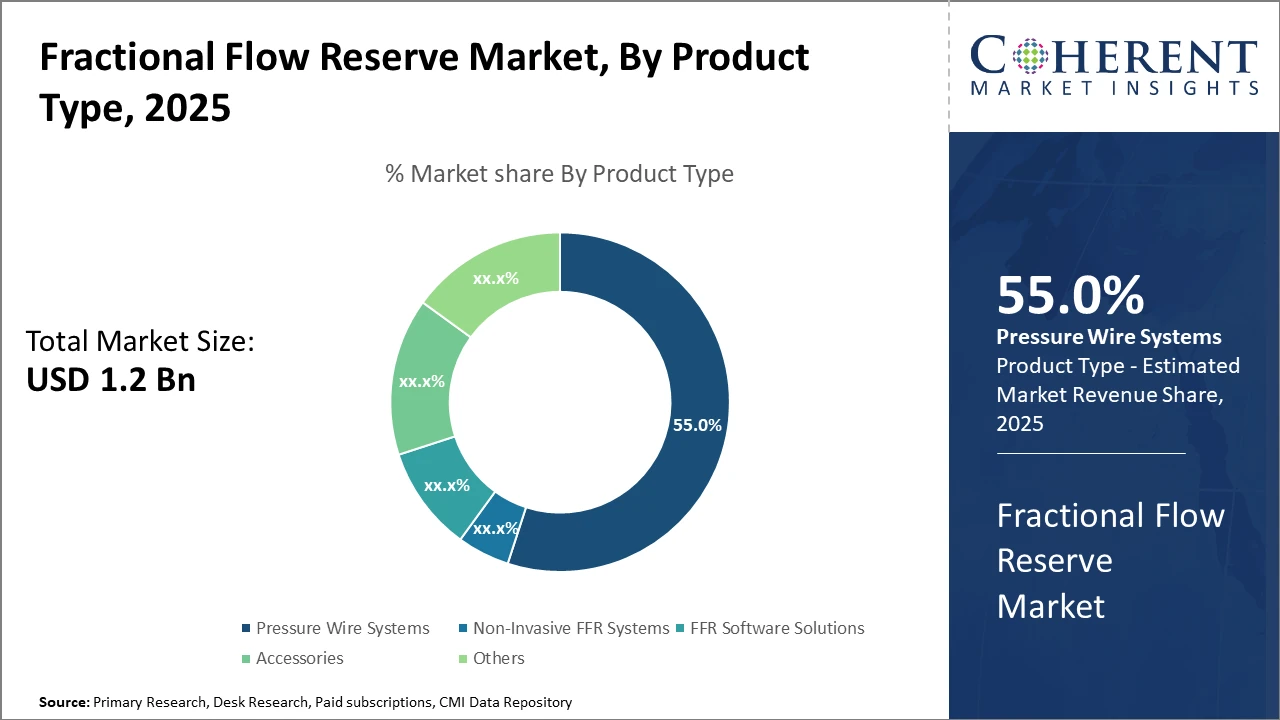
The Pressure Wire Systems segment dominates the Fractional Flow Reserve market, accounting for over 55% industry share, attributed to its established technological reliability and extensive clinical validation.
Non-Invasive FFR Systems represent the fastest-growing subsegment, driven by rising demand for patient-friendly diagnostic procedures.
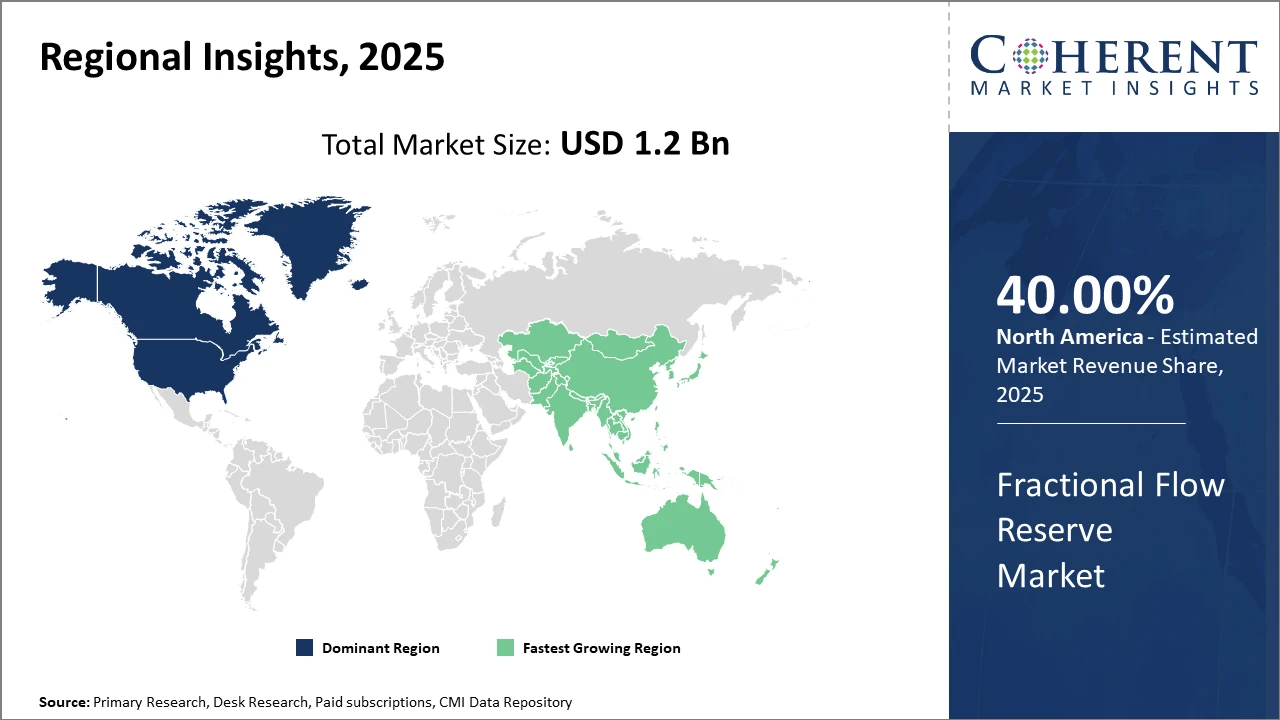
Geographically, North America holds the largest industry share, supported by a well-established healthcare ecosystem and the presence of key market players.
Meanwhile, the Asia Pacific region demonstrates the highest CAGR, fueled by expanding healthcare infrastructure investments and the rising prevalence of cardiovascular diseases.
Europe maintains steady growth through continuous reimbursement reforms and technological adoption in cardiac care centers.
Fractional Flow Reserve Market Segmentation Analysis
To learn more about this report, Download Free Sample
Fractional Flow Reserve Market Insights, By Product Type
Pressure Wire Systems remain the backbone of Fractional Flow Reserve diagnostics due to their proven accuracy and extensive clinical validation across cardiology centers worldwide. This subsegment’s dominance stems from continuous innovations, such as the integration of wireless technology and refined sensor materials, improving measurement reliability and user experience. Concurrently, Non-Invasive FFR Systems demonstrate the fastest segmental growth as they reduce patient risk and procedural time, responding to the increased demand for less intrusive diagnostic methods. These systems leverage CT angiography data to calculate flow reserve, expanding market scope, particularly in outpatient settings. FFR Software Solutions facilitates data analysis and interpretation, aiding cardiologists in treatment planning, but currently occupies a smaller market share due to integration challenges.
Fractional Flow Reserve Market Insights, By Application
Stable Coronary Artery Disease accounts for the majority of FFR applications due to the high prevalence of chronic ischemic conditions requiring detailed physiological assessment. The subsegment benefits from established clinical guidelines recommending FFR use to guide treatment decisions, resulting in widespread adoption, particularly in hospitals and diagnostic centers. Acute Coronary Syndrome represents the fastest-growing application as clinicians increasingly utilize FFR to distinguish lesion significance during emergent interventions, prompted by recent studies validating its safety and efficacy in unstable settings. Other Cardiovascular Applications include peripheral artery disease and valvular heart conditions, where FFR shows emerging potential. The ‘Others’ subsegment covers less frequent or investigational applications supported by clinical trials.
Fractional Flow Reserve Market Insights, By End-User
Hospitals remain the primary end-users for Fractional Flow Reserve owing to their comprehensive cardiology departments, higher procedural volumes, and capacity to invest in advanced diagnostic equipment. The dominance is driven by rapid technology uptake and reimbursement facilitation in inpatient settings. Ambulatory Surgical Centers are the fastest-growing end-user subsegment, benefiting from procedural shifts towards outpatient care combined with improvements in minimally invasive FFR devices and streamlined workflows. Cardiac Surgery Clinics and Diagnostic Centers hold significant shares as specialized facilities instrumental in patient diagnosis and elective interventions.
Fractional Flow Reserve Market Trends
The Fractional Flow Reserve market is witnessing several disruptive trends shaping future trajectories.
The push towards non-invasive FFR techniques such as FFR derived from CT angiography (FFRCT) is becoming prominent, as demonstrated by a 25% increase in FFRCT utilization in the U.S. during 2025.
This development not only reduces procedural risk but also increases diagnostic throughput in cardiac centers.
Another significant trend involves the incorporation of AI and machine learning algorithms to augment decision-making during FFR assessments, improving diagnostic accuracy by approximately 18% in clinical evaluations recorded in Europe in 2024.
Lastly, value-based healthcare models championing cost-effective treatments promote market growth by incentivizing the adoption of FFR to avoid unnecessary interventions.
Fractional Flow Reserve Market Insights, By Geography
To learn more about this report, Download Free Sample
North America Fractional Flow Reserve Market Analysis and Trends
In North America, the dominance of the Fractional Flow Reserve market is underscored by a robust healthcare infrastructure, advanced R&D capabilities, and the strong presence of leading market companies, which collectively contribute over 40% industry share. The U.S. accounts for the largest portion due to its high procedural volumes, reimbursement support, and early adoption of innovative FFR technologies.
Asia Pacific Fractional Flow Reserve Market Analysis and Trends
Meanwhile, Asia Pacific exhibits the fastest growth with a CAGR nearing 12%, driven by increasing cardiovascular disease prevalence, expanding hospital infrastructure, and government focus on cardiac care. Countries like China and India are spearheading this growth, supported by rising healthcare expenditure and improving access to advanced diagnostics.
Fractional Flow Reserve Market Outlook for Key Countries
USA Fractional Flow Reserve Market Analysis and Trends
The U.S. market remains the most significant contributor with extensive use of FFR in diagnostic cardiology and PCI guidance. Several high-profile cardiovascular centers, supported by reimbursement policies from Centers for Medicare & Medicaid Services (CMS), reported a 15% increase in FFR procedures during 2024. Leading players such as Abbott Laboratories and Philips Healthcare maintain dominant market positions by introducing advanced pressure wire systems and AI-driven software, influencing the market dynamics significantly.
China Fractional Flow Reserve Market Analysis and Trends
China’s market growth is propelled by rapidly expanding healthcare infrastructure and government initiatives aimed at cardiovascular disease management. 2025 witnessed a surge in cardiac catheterization procedures by over 18%, facilitating increased adoption of FFR technologies. Local manufacturers are collaborating with international firms to improve accessibility and affordability, fostering competitive yet growth-oriented market conditions.
Analyst Opinion
Supply-side dynamics indicate that advancements in pressure-sensor technology have significantly increased production capacity in 2024, driving down costs and improving market accessibility. For instance, major launches of next-generation FFR pressure wires reduced per-unit costs by up to 12%, enhancing adoption in both emerging and developed markets.
On the demand front, the growing use of FFR in catheterization labs to guide percutaneous coronary interventions (PCI) demonstrates a shift towards precision cardiology. Recent clinical data from 2024 showed a 15% increase in FFR-guided PCI procedures globally, highlighting demand diversification across cardiology centers.
Micro-indicators point to increased import volumes of FFR-compatible diagnostic devices in countries like India and Brazil, with import growth rates of 18% and 21% respectively in the last fiscal year, reflecting expanding emerging market penetration.
Nano-scale indicators reveal increasing utilization of wireless FFR measurement systems, which accounted for a 9% market share in 2025, due to minimized patient discomfort and enhanced procedural efficiency as seen at several leading cardiovascular centers in Europe in 2023.
Market Scope
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2025 | Market Size in 2025: | USD 1.2 billion |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 8.5% | 2032 Value Projection: | USD 2.05 billion |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: | Terumo Corporation, Volcano Corporation, Biosensors International Group, OrbusNeich Medical, Nihon Kohden Corporation, Merit Medical Systems, St. Jude Medical, MicroPort Scientific Corporation, Acist Medical Systems, Zoll Medical Corporation. | ||
| Growth Drivers: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Fractional Flow Reserve Market Growth Factors
The growing prevalence of coronary artery disease worldwide fuels the need for precise diagnostic tools like FFR, directly impacting market growth. According to the American Heart Association, cardiovascular diseases accounted for nearly 31% of global deaths in 2023, necessitating more robust intervention strategies leveraging FFR. Technological advancements in non-invasive and wireless FFR devices improve patient comfort and clinical workflow efficiency, supporting wider clinical acceptance. Rising healthcare expenditure in emerging economies such as India and China, coupled with government initiatives to upgrade cardiovascular infrastructure, significantly expands market scope and revenue potential. Furthermore, the increasing number of cardiac catheterization procedures globally, registering an 8% annual growth rate in 2024, drives persistent demand for reliable FFR systems.
Fractional Flow Reserve Market Development
In June 2025, MedHub-AI’s AutocathFFR® AI-powered software received PMDA clearance in Japan, with Terumo appointed as the distribution partner, enabling wider adoption of non-invasive, AI-driven fractional flow reserve (FFR) assessment. The approval marked a significant step toward reducing procedure time and reliance on invasive pressure wires in coronary diagnostics.
In November 2024, Boston Scientific launched Avvigo, a multi-functional cardiovascular system designed to enhance procedural efficiency and imaging accuracy across complex coronary interventions. The platform integrated advanced visualization and workflow tools, supporting improved decision-making during interventional cardiology procedures.
In October 2023, Philips confirmed that instantaneous wave-free ratio (iFR) and fractional flow reserve (FFR) deliver equivalent clinical effectiveness, providing physicians with greater flexibility in selecting diagnostic approaches for coronary artery disease. The announcement reinforced confidence in physiology-guided assessment strategies and supported broader adoption of wire-free diagnostic options.
Key Players
Leading Companies of the Market
Terumo Corporation
Volcano Corporation
Biosensors International Group
OrbusNeich Medical
Nihon Kohden Corporation
Merit Medical Systems
St. Jude Medical
MicroPort Scientific Corporation
Acist Medical Systems
Zoll Medical Corporation
Among these market players, several have employed competitive strategies such as expanding their product portfolios through R&D investments and strategic acquisitions. Notably, Abbott Laboratories' acquisition of vascular imaging technology assets in 2024 enhanced its position in the FFR market, resulting in a 10% increase in market penetration in key regions. Similarly, Philips Healthcare launched AI-enabled FFR software in 2023, improving diagnostic accuracy by approximately 18% and thereby increasing its customer base across Europe and Asia. Boston Scientific’s focus on integrated FFR and intravascular imaging platforms has propelled its revenue growth by 12% in North America since the prior year.
Fractional Flow Reserve Market Future Outlook
The future of the FFR market is characterized by a transition toward non-hyperemic and non-invasive technologies such as angiography-derived FFR and CT-based FFR solutions. These innovations are expected to broaden clinical adoption by offering faster, radiation-efficient, and drug-free procedures. AI integration into imaging platforms will enhance diagnostic accuracy and broaden accessibility in emerging markets. With cardiovascular diseases rising globally, FFR is expected to become a standard diagnostic tool before PCI, expanding adoption across hospitals and ambulatory care settings.
Fractional Flow Reserve Market Historical Analysis
Historically, the FFR market expanded as interventional cardiology shifted from purely anatomical to physiology-based decision-making. In the early 2000s, clinical trials proved that FFR-guided PCI produced better patient outcomes, reducing unnecessary stenting and improving long-term prognosis. Through the 2010s, adoption accelerated as hospitals invested in advanced catheterization lab technologies and pressure wire systems. Reimbursement improvements in major markets, along with growing global cardiovascular disease burden, strengthened commercial uptake.
Sources
Primary Research Interviews:
Cardiologists
Interventional Cardiology Specialists
Hospital Procurement Officers
Databases:
PubMed Clinical Trials
FDA MAUDE
IQVIA Medical Devices
WHO Cardiology Data
Magazines:
Cardiology Today
MedTech Dive
Diagnostic Imaging Magazine
Journals:
Circulation
JACC (Journal of the American College of Cardiology)
European Heart Journal
Newspapers:
NYT Health
WSJ Healthcare
Reuters Medical News
Associations:
American College of Cardiology (ACC)
European Society of Cardiology (ESC)
AHA
Share
Share
About Author
Manisha Vibhute is a consultant with over 5 years of experience in market research and consulting. With a strong understanding of market dynamics, Manisha assists clients in developing effective market access strategies. She helps medical device companies navigate pricing, reimbursement, and regulatory pathways to ensure successful product launches.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients