Global foot and mouth disease vaccine market is estimated to be valued at USD 3.15 Bn in 2025 and is expected to reach USD 5.66 Bn by 2032, exhibiting a compound annual growth rate (CAGR) of 8.7% from 2025 to 2032.
Discover market dynamics shaping the industry: Download Free Sample
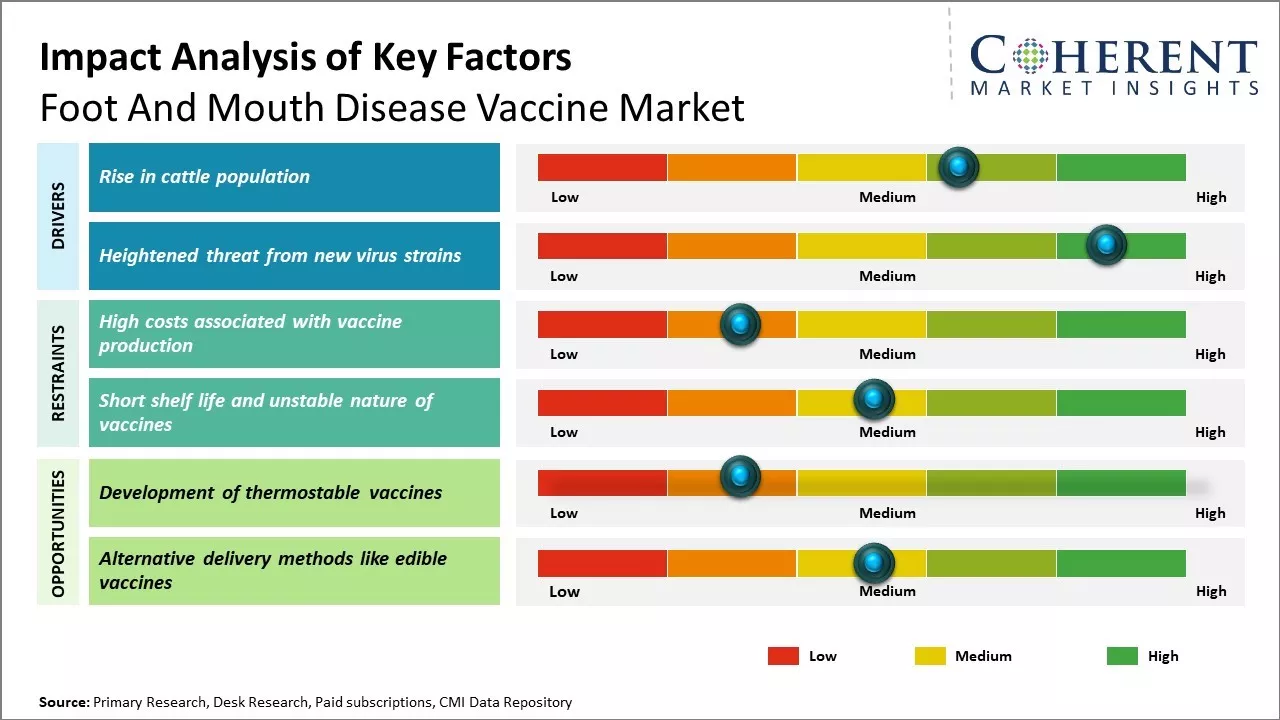
Huge presence of livestock and increasing demand for animal-derived food products can drive the market growth. The supportive government initiatives to curb outbreaks of this highly contagious disease can also drive the market growth. Key market players are investing in research & development activities for developing effective foot and mouth vaccines. However, high costs associated with vaccine production and storage can hamper the market growth. The mitigation of huge economic losses due to FMD outbreaks can boost demand for foot and mouth disease vaccines.
Rise in cattle population
Global cattle population has been steadily increasing over the past few decades to meet rising demand for dairy and meat products. As cattle are highly susceptible to foot and mouth disease, growing number of cattle can raise the risk of FMD outbreaks. Developing regions like Asia Pacific and Latin America that are witnessing a surge in livestock farming activities have emerged as major markets for FMD vaccines. Moreover, the commercialization of cattle rearing practices has led to clustering of cattle in large farms, which amplifies the spread of the disease. Controlling FMD epidemics through mass vaccination programs has become a priority for governments to minimize disruption in cattle trade and international movement of animals. This growing at-risk cattle population base leads to increased off-take and demand for effective FMD vaccines. In nations with substantial livestock populations like China and Mexico, the risk of infection is heightened. In September 2021, according to the Food and Agriculture Organization (FAO) Report, India's national cattle herd was 306.7 million animals in 2022, marking an increase of 1.2 million head from Italy's 2021 cattle count.
Get actionable strategies to beat competition: Download Free Sample
Heightened threat from new virus strains
Foot and mouth disease virus has a high mutation rate which allows new variants to evolve constantly. Emergence of novel strains that the existing vaccines may not offer protection against can pose a serious challenge. In recent years, unusual outbreaks caused by atypical FMD virus strains have been reported from parts of Asia, Africa and South America. For example, a virus type O pandemic strain affected huge cattle herds in countries like South Korea, Vietnam and Taiwan in 2010-2011 despite vaccination. Similarly, an unusual topotype of type A virus caused repeated epidemics in Middle East and North Africa since 2009 even in vaccinated cattle. Containing such rapidly evolving strains with cross-border spread capabilities requires continuous vaccine development and shortening of response times. This evolving epidemiological pattern boosts the need for enhanced surveillance of FMD viruses to design vaccines matching the circulating field strains more precisely.
Key Takeaways from Analyst:
Global foot and mouth disease vaccine market growth is driven by increasing outbreaks of FMD virus across various regions. Livestock farming plays an important role in many developing economies and FMD poses a major threat to this industry. Maintaining an effective vaccination program is critical to prevent economic losses stemming from outbreaks. Several countries in Asia Pacific and Latin America that have large cattle populations are expected to significantly boost demand. Vaccine manufacturers are also investing in R&D to develop more effective vaccines with single dose regimen and thermostability at higher temperatures suited for tropical conditions. However, factors such as high costs associated with large scale vaccination programs can hamper the market growth. Poorly implemented vaccination strategies and lack of necessary infrastructure in many developing and underdeveloped regions can hamper the market growth. Furthermore, alternatives such as movement control strategies during outbreaks reduce dependence on repeat vaccination. The dominance of a few major players also limits opportunities for local manufacturers.
Market Challenges: High costs associated with vaccine production
High costs associated with vaccine production can hamper the global foot and mouth disease vaccine market growth. Producing FMD vaccines involves a complex and costly process. FMD viruses have multiple serotypes and each serotype requires its own dedicated vaccine. Manufacturers must conduct extensive research and testing to develop vaccines for each new emerging strain. They also need to continuously monitor the circulating strains and update the vaccines as needed. The manufacturing process itself is also risky and expensive. FMD viruses can only be cultivated in live animals under high biosecurity conditions. Vaccine production involves handling the live viruses and isolating them from infected tissues. Ensuring biosecurity and safety during every step increases production costs tremendously. Additional costs are incurred in stability testing, validation, sterilization and meeting stringent global quality standards. With seven distinct serotypes of FMD viruses known to exist worldwide, continuous vaccine development and strain surveillance further increase costs.
Market Opportunities: Development of thermostable vaccines
The development of thermostable vaccines provides a unique opportunity for global foot and mouth disease vaccine market growth. Currently, cold chain storage and distribution requirements limit the efficacy and practicality of existing FMD vaccines in many parts of the world, especially developing regions where the disease remains prevalent. Thermostable vaccines that do not require consistent refrigeration would eliminate these cold chain constraints and allow for more widespread FMD control programs globally. With the ability to effectively and reliably transport and administer vaccines at higher ambient temperatures, thermostable FMD vaccines could enable vaccination campaigns in remote and rural agricultural communities that currently have little to no access to cold storage facilities. This would help reduce the risks of FMD outbreaks in newly immunized populations and aid efforts for disease eradication. Transporting and stockpiling thermostable vaccines would also be significantly less complex and costly compared to conventional cold-chain dependent vaccines. The cost savings from simplified logistics could be reallocated to increasing vaccine coverage in developing nations.
Discover high revenue pocket segments and roadmap to it: Download Free Sample
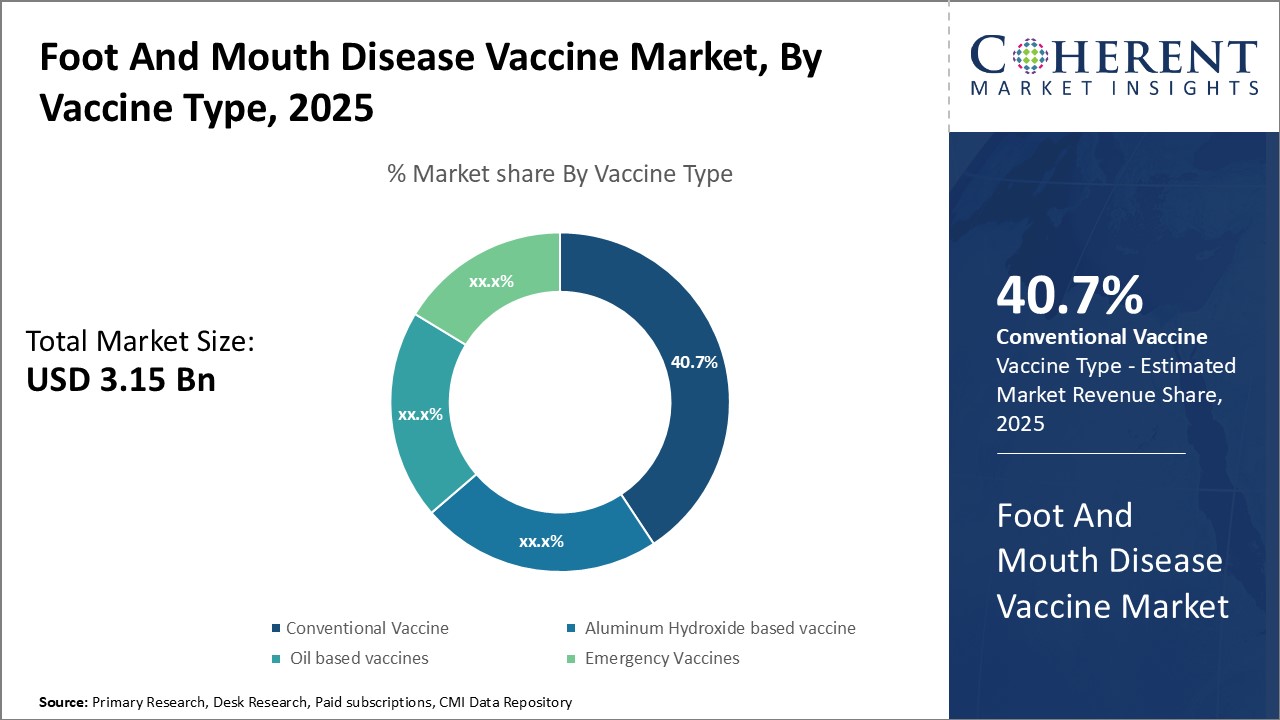
By Vaccine Type: Conventional vaccine lead due to strong efficacy against multiple variants
Among vaccine type, conventional vaccine segment is estimated to contribute the highest market share of 40.7% in 2025 due to its ability to provide broad protection against multiple virus variants. Conventional vaccines contain inactivated whole virus particles which elicit strong antibody responses against various antigenic sites on the virus. This allows the vaccines to be effective against different serotypes and strains that may emerge in a region over time. Outbreaks can involve several strains simultaneously, and conventional vaccines offer cross-protection that helps control such mixed infections better than other alternatives. The multivalent nature of conventional vaccines provides them versatility that appeals to farmers and veterinary bodies. Only one or two doses are needed to protect cattle, sheep, goats and pigs from the major variants circulating in a particular country or continent. This simplifies vaccination programs as compared to needing multiple specific vaccines. Storage and logistics are also easier as fewer products must be kept in stock. Overall, conventional vaccines provide the best value for mass vaccination campaigns aimed at population-level disease prevention.
By Animal Type: Cattle dominate due to high susceptibility and economic impacts
Among animal type, cattle segment is estimated to hold the highest market share of 50.1 % in 2025. Cattle are highly susceptible to foot and mouth disease virus (FMDV) infection and experience severe symptoms such as blisters inside the mouth and on the feet. Even subclinical infections in cows can cause drops in milk yield by as much as 30-40.5%. Moreover, outbreaks in cattle herds have devastating economic consequences for farmers. Loss of productivity from infected cattle can damage livelihoods. Slaughter restrictions during outbreaks also disrupt beef export and processing. The costs of control measures, culling infected animals, and trade embargoes amount to billions each year according to industry figures. W Countries prioritize vaccinating this species as the main defense against FMDV spread. Breeding herds are usually vaccinated regularly to maintain population immunity levels. Young stock is vaccinated before being exposed to potential infection at crowded livestock markets and prior to exporting. The large cattle populations make mass vaccination programs logistically challenging but critical for early disease detection and rapid response. Suitable vaccines must be available in adequate doses to vaccinate millions of heads of cattle annually in at-risk regions.
Need a Different Region or Segment? Download Free Sample
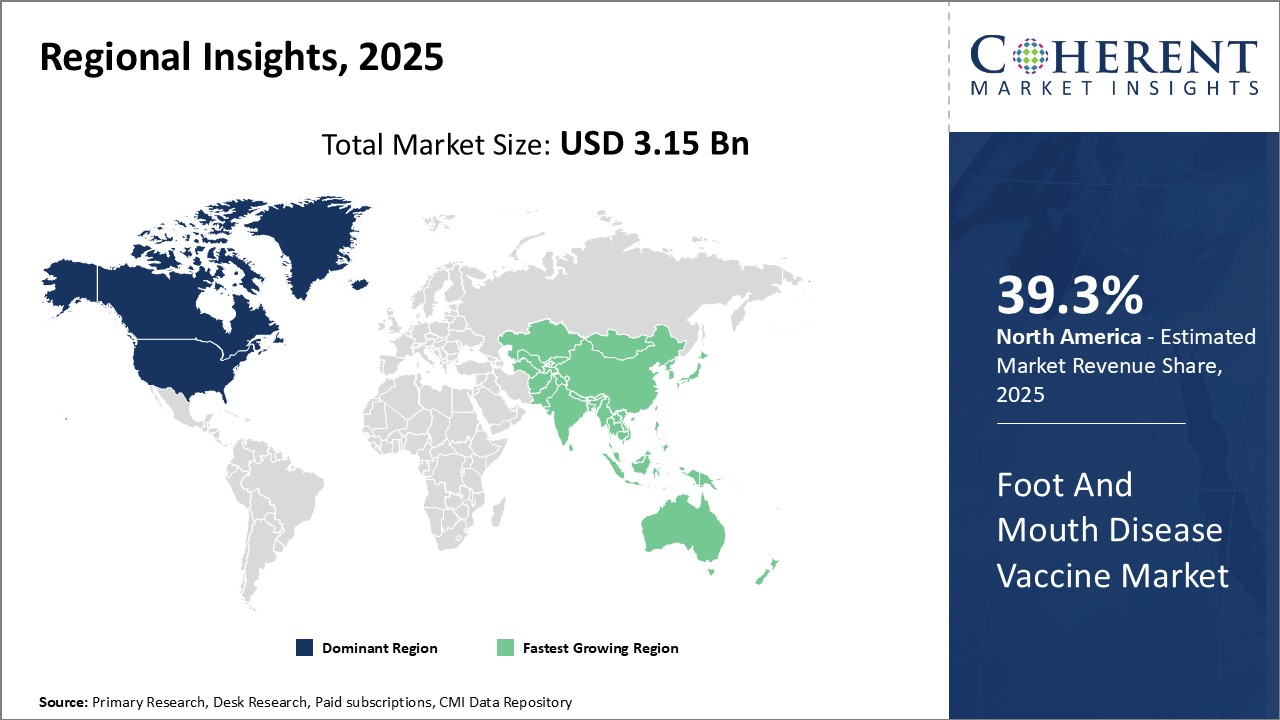
North America remains the dominant region in the global foot and mouth disease vaccine market with estimated market share of 39.3% in 2025. The U.S. accounts for the majority of the market share due to strong presence of leading vaccine manufacturers in the country. Strong support from the government agencies for livestock healthcare and favorable regulations have boosted vaccine adoption among farmers. Furthermore, the high-quality certifications for vaccines by regulatory bodies ensures consumer safety and confidence. The North American Free Trade Agreement has also enhanced exports of American vaccines to Mexico and Canada over the years.
Asia Pacific has emerged as the fastest growing regional market for foot and mouth disease vaccine globally. Rapid expansion of the dairy and meat industries together with rising livestock counts in major countries like India and China are driving increased prevention through vaccination. To protect their massive cattle population and meet the burgeoning demand for milk and meat, the APAC governments are focused on extensive vaccination programs. This has lured multinational corporations to establish production facilities and collaborate with local players. Counties like India often have price control mechanisms in place to ensure affordability of vaccines for small farmers. This has made vaccines highly accessible and supported strong revenue growth of regional and global participants.
Foot and Mouth Disease Vaccine Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 3.15 Bn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 8.7% | 2032 Value Projection: | USD 5.66 Bn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
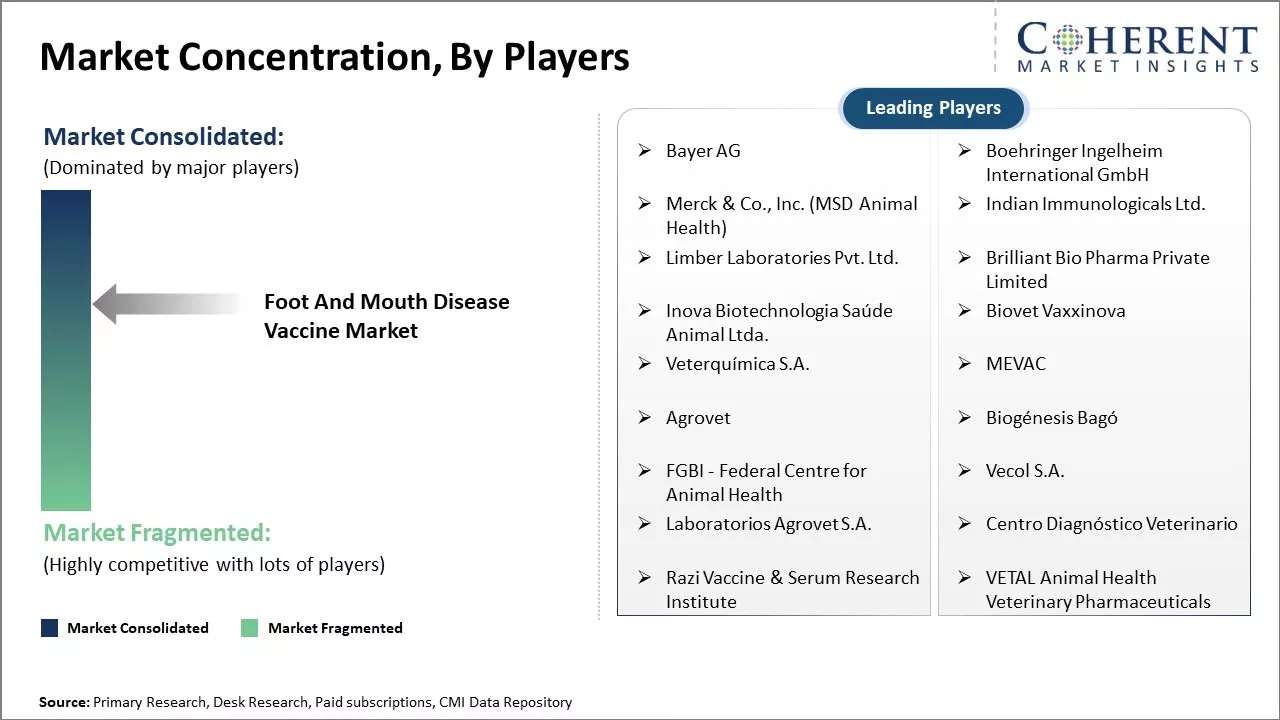
| Companies covered: |
Bayer AG, Boehringer Ingelheim International GmbH, Merck & Co., Inc. (MSD Animal Health), Indian Immunologicals Ltd., Limber Laboratories Pvt. Ltd., Brilliant Bio Pharma Private Limited, Inova Biotechnologia Saúde Animal Ltda., Biovet Vaxxinova, Veterquímica S.A., MEVAC, Agrovet, Biogénesis Bagó, FGBI - Federal Centre for Animal Health, Vecol S.A., Laboratorios Agrovet S.A., Centro Diagnóstico Veterinario, Razi Vaccine & Serum Research Institute, VETAL Animal Health Veterinary Pharmaceuticals |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Share
Share
About Author
Vipul Patil is a dynamic management consultant with 6 years of dedicated experience in the pharmaceutical industry. Known for his analytical acumen and strategic insight, Vipul has successfully partnered with pharmaceutical companies to enhance operational efficiency, cross broader expansion, and navigate the complexities of distribution in markets with high revenue potential.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients