Foot And Ankle Devices Market Size and Forecast – 2025 – 2032
The Global Foot and Ankle Devices Market size is estimated to be valued at USD 4.6 billion in 2025 and is expected to reach USD 7.9 billion by 2032, exhibiting a compound annual growth rate (CAGR) of 7.8% from 2025 to 2032.
Global Foot And Ankle Devices Market Overview
Foot and ankle device products include fixation plates and screws, intramedullary nails, external fixators, ankle arthroplasty implants, total ankle replacement systems, fusion devices (cages and bone graft substitutes), tendon repair implants, orthobiologics, and minimally invasive deformity-correction instruments. Offloading products such as braces, walking boots, orthoses and joint-sparing implants support conservative management. Surgical instruments encompass specialized jigs, reamers, and navigation guides tailored to small-bone anatomy. Recent product innovations include patient-specific 3D-printed implants for complex reconstructions, porous titanium surfaces to enhance osseointegration, low-profile locking plate systems for osteoporotic bone, and ankle replacement designs that better replicate native kinematics.
Key Takeaways
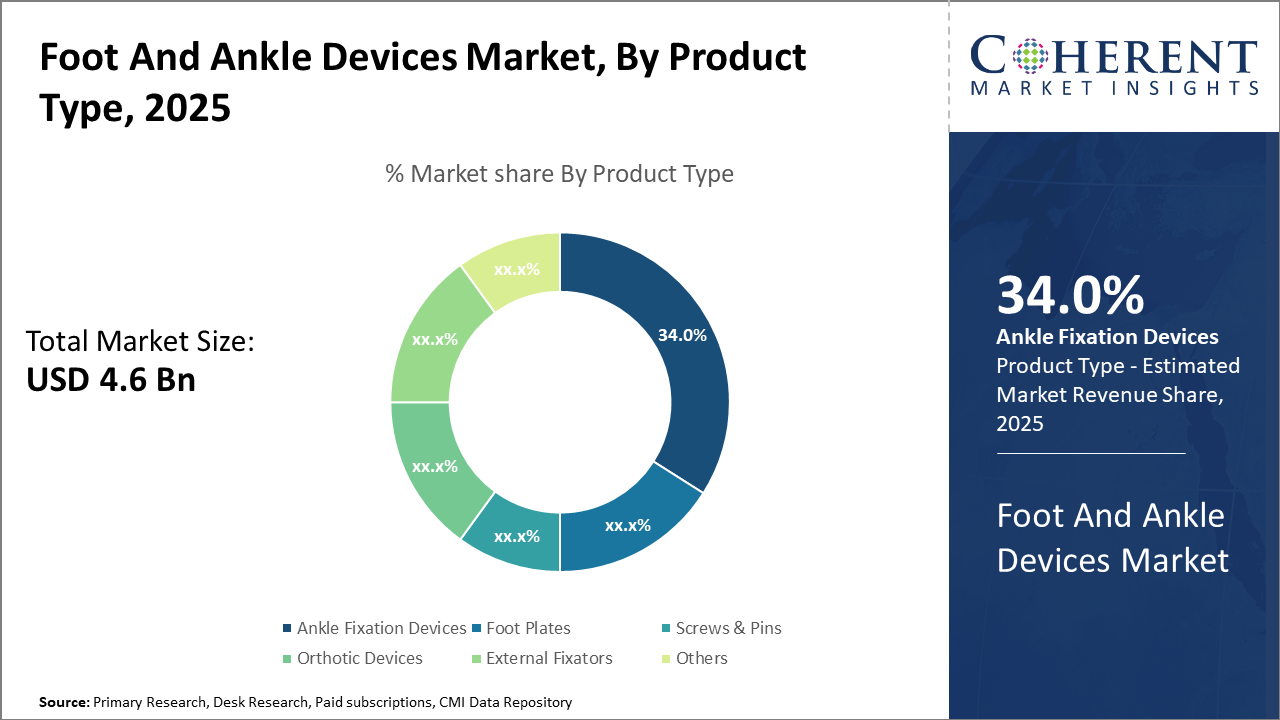
Key segment-based takeaways reveal that ankle fixation devices continue to dominate the Foot and Ankle Devices Market size with a 34% share, driven by the high prevalence of ankle fractures globally.
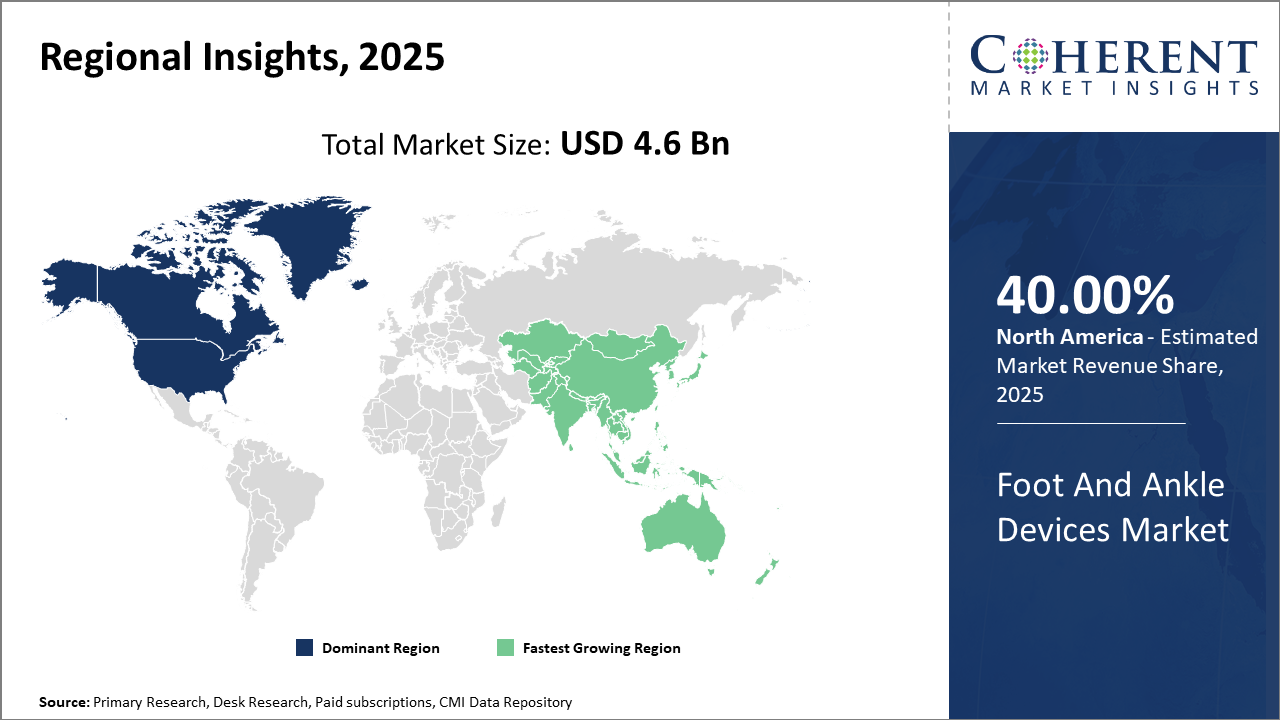
From a regional standpoint, North America remains the dominant region for market revenue, accounting for approximately 40% of the total industry share due to robust healthcare infrastructure and an advanced orthopedic ecosystem.
Meanwhile, the Asia Pacific exhibits the fastest market growth, driven by increasing healthcare investments and rising awareness of orthopedic conditions in countries such as China and India.
Foot And Ankle Devices Market Segmentation Analysis
To learn more about this report, Download Free Sample
Foot And Ankle Devices Market Insights, By Product Type
Ankle Fixation Devices lead due to the high incidence of ankle fractures and ligament injuries, facilitating critical stabilization during recovery. These devices are preferred for their biomechanical strength and improved surgical outcomes, with current trends emphasizing minimally invasive fixation techniques that reduce operation time and post-surgical complications. Foot Plates, Screws & Pins represent the fastest growing segment, propelled by innovations in bioabsorbable materials that minimize the need for secondary surgeries to remove implants. Foot Plates aid in restoring structural integrity and load distribution, while screws & pins provide versatile options for various fracture types.
Foot And Ankle Devices Market Insights, By Application
Trauma & Fracture Repair remains the cornerstone due to the high frequency of traumatic injuries worldwide, necessitating immediate and effective interventions. Focused innovations in locking plate technologies and cannulated screws provide superior fixation for complicated fractures. Arthrodesis & Joint Fusion are the fastest growing applications propelled by rising osteoarthritis prevalence, with advanced fusion devices enabling pain relief and joint stabilization, often preferred over joint replacements in certain demographics. Deformity Correction devices assist in addressing congenital and acquired angular deformities, with growing utilization of minimally invasive implants to enhance precision.
Foot And Ankle Devices Market Insights, By End-User
Hospitals retain dominance due to their comprehensive infrastructure and capacity to perform complex foot and ankle surgeries requiring advanced implant systems and post-op care. Ambulatory Surgical Centers emerge as the fastest growing segment, boosted by patient preference for outpatient interventions and cost-effectiveness, especially for elective procedures like elective osteotomies and orthotic fittings. Orthopedic Clinics and Specialty Clinics maintain significant roles in follow-ups, conservative management involving orthotics, and minor surgical interventions.
Foot And Ankle Devices Market Trends
The Foot and Ankle Devices market has seen notable trends shaping its landscape.
Firstly, the rise of minimally invasive and robotic-assisted surgeries has significantly enhanced procedural accuracy and postoperative recovery, with U.S.-based hospitals reporting an 18% uptick in minimally invasive ankle surgeries in 2024.
Secondly, additive manufacturing technologies, especially 3D-printed implants tailored for complex anatomies, gained traction with Europe witnessing adoption rates increased by 20% in 2025, led by Germany and France.
Lastly, digitization and telehealth integration offer new avenues for post-surgical care, especially in the Asia Pacific, which reported over 30% growth in virtual monitoring of orthopedic patients in 2025.
Foot And Ankle Devices Market Insights, By Geography
To learn more about this report, Download Free Sample
North America Foot And Ankle Devices Market Analysis and Trends
In North America, the dominance in the Foot and Ankle Devices market is attributed to advanced healthcare infrastructure, widespread orthopedic expertise, and active participation of key market companies like Stryker and Zimmer Biomet. The region commands roughly 40% of the market share, bolstered by large volumes of sports injury surgeries and geriatric care. U.S. regulatory policies also support accelerated device approvals, aiding rapid product launches.
Asia Pacific Foot And Ankle Devices Market Analysis and Trends
Meanwhile, Asia Pacific exhibits the fastest growth with a CAGR exceeding 10% during the forecast period. Factors such as increasing healthcare expenditure, growing geriatric population, rise in diabetic foot ulcers, and government incentives for medical device innovation in China and India fuel this expansion. Notable entities, including Medtronic and Smith & Nephew, have intensified penetration efforts here by partnering with local distributors to enhance market reach.
Foot And Ankle Devices Market Outlook for Key Countries
USA Foot And Ankle Devices Market Analysis and Trends
The USA's market is pivotal given its advanced orthopedic ecosystem and higher prevalence of sports injuries necessitating sophisticated foot and ankle devices. In 2024, the country recorded over 250,000 ankle fixation surgeries, showcasing significant market uptake. Major players like Stryker, Zimmer Biomet, and DePuy Synthes are advancing product innovation with integrated sensor technologies and minimally invasive instrumentation. These strategic developments have increased both market share and revenue streams, making the USA a linchpin in market dynamics.
Germany Foot And Ankle Devices Market Analysis and Trends
Germany's Foot and Ankle Devices market is characterized by high adoption of cutting-edge orthopedic implants bolstered by a strong healthcare insurance system. The country has witnessed a 12% rise in joint fusion procedures involving ankle and foot devices in 2024, indicating robust demand. Leading companies emphasize partnerships with top orthopedic clinics and universities for clinical trials, accelerating technology validation and acceptance. This collaborative model strengthens Germany’s position as a hub for orthopedic innovation in Europe.
Analyst Opinion
Increasing demand for ankle fixation devices reflects the rise in traumatic foot and ankle injuries. For instance, U.S. hospital data recorded a 12% increase in surgical ankle fixation procedures in 2024 compared to 2023, signaling robust demand growth. Price stabilization of titanium and bioabsorbable materials, key for manufacturing implants, has improved profit margins, with pricing declines by 3% globally in early 2025 supplying manufacturers with cost advantages.
The surge in outpatient surgical centers is amplifying the accessibility of foot and ankle device procedures. Asia Pacific alone experienced a 15% uptick in outpatient orthopedic procedures using such devices in 2024, encouraging market players to expand presence in these regions. Import volumes of foot and ankle implants into Europe grew by 8.6% in the first half of 2025, driven largely by increasing surgeries related to sports injuries.
Demand-side dynamics are also influenced by aging populations. For example, Japan reported a 17% increase in elderly patients receiving foot and ankle joint replacement surgery in 2024, underscoring the growing use cases in geriatric orthopedics. Simultaneously, growth in diabetic foot ulcer cases contributes to higher utilization of specialized wound healing devices within the market.
Micro-indicators reveal that advancements in 3D printing technologies for customizable orthotic devices are reshaping product offerings. Notably, a leading implant manufacturer launched patient-specific devices in 2025, reducing implant failure rates by 6% in early clinical assessments. This technological evolution is expected to fuel segment revenue expansion significantly.
Market Scope
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2025 | Market Size in 2025: |
USD 4.6 billion |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 7.8% | 2032 Value Projection: |
USD 7.9 billion |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: | Smith & Nephew plc, Stryker Corporation, Zimmer Biomet Holdings, Inc., Medtronic plc, Integra LifeSciences Holdings Corporation, Wright Medical Group N.V., ConforMIS, Inc., Arthrex, Inc., DJO Global, Inc., Össur hf., MicroPort Scientific Corporation. | ||
| Growth Drivers: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Foot And Ankle Devices Market Growth Factors
Key drivers include rising incidence of foot and ankle injuries due to sports and aging demographics, medical advancements in implant materials such as bioabsorbable polymers and titanium alloys, increasing adoption of minimally invasive surgeries, and enhanced healthcare expenditure, particularly in emerging economies. For instance, the prevalence of sports-related ankle injuries increased by 9% globally in 2024, driving demand for fixation devices. Moreover, government-funded programs promoting advanced orthopedic care in the Asia Pacific boosted market revenue by 14% in 2025. Growing awareness and treatment of diabetic foot ulcers also supported expansion, notably with wound care implants that provided an alternative solution, reducing surgical complications.
Foot And Ankle Devices Market Development
In February 2024, Restor3d expanded its orthopedic portfolio with patient-specific implants, including a Total Talus Replacement system and a Kinos Axiom line extension. These launches leverage Restor3d’s proprietary 3D-printing and biomaterial design technologies to deliver fully anatomical, customized implants that enhance joint congruency, stability, and long-term functional outcomes. The Total Talus Replacement system addresses complex ankle pathologies where conventional reconstruction is inadequate, while the Kinos Axiom expansion provides improved modularity and anatomic fidelity for midfoot and forefoot procedures.
In March 2024, Stryker strengthened its joint replacement portfolio through the acquisition of SERF SAS, a French orthopedic implant manufacturer with expertise in hip prostheses. The acquisition brings SERF’s established products—most notably the NOVAE® acetabular cup system—into Stryker’s reconstructive lineup, enhancing its global capabilities in primary and revision hip arthroplasty.
Key Players
Leading Companies of the Market
Smith & Nephew plc
Stryker Corporation
Zimmer Biomet Holdings, Inc.
Medtronic plc
Integra LifeSciences Holdings Corporation
Wright Medical Group N.V.
ConforMIS, Inc.
Arthrex, Inc.
DJO Global, Inc.
Össur hf.
MicroPort Scientific Corporation
Competition focuses heavily on expanding product portfolios through R&D investments and strategic acquisitions. For example, Stryker’s 2024 acquisition of an orthopedic 3D printing company expanded its patient-specific implant capabilities, resulting in a reported 11% increase in segment revenue within the first six months post-merger. Similarly, DePuy Synthes focused on integrating minimally invasive surgical instruments with improved implant designs, boosting its North American market share in 2025 by approximately 5%.
Foot And Ankle Devices Market Future Outlook
In the coming years, the foot & ankle market will accelerate toward patient-specific care, motion-preserving technologies, and integrated perioperative ecosystems. 3D-printing of porous, patient-matched implants will enable complex reconstructions with improved osseointegration and fit for deformity correction and tumor resection defects. Total ankle arthroplasty designs will continue to refine biomechanics and fixation strategies to extend implant longevity and expand indications beyond low-demand patients. Sensor-enabled implants and instrumented orthoses that provide real-time load and gait feedback will support rehabilitation and allow earlier detection of implant overload or failure. Minimally invasive, percutaneous fixation systems and biologic enhancements (cell therapy scaffolds, growth-factor release systems) may reduce the need for large fusion constructs and preserve joint mobility when clinically appropriate.
Foot And Ankle Devices Market Historical Analysis
Foot and ankle device technology evolved from the adaptation of general orthopedic fixation hardware to a specialized, anatomically focused set of implants and instruments reflecting the complex biomechanics of the hindfoot and forefoot. Historically, surgeons repurposed plates and screws intended for larger bones; as understanding of foot kinematics and pathology increased, dedicated low-profile locking plates, miniaturized intramedullary nails, specially contoured fixation systems, and an array of arthrodesis devices were developed to address unique loading patterns and the need for accurate alignment. The development of total ankle arthroplasty—initially challenged by early implant failures—progressed with improved bearing designs, fixation surfaces, and polyethylene inserts; iterative design and materials advances (cross-linked polyethylenes, porous titanium surfaces) gradually improved survivorship.
Sources
Primary Research Interviews:
Podiatric surgeons
Orthopedic foot & ankle specialists
Implant design engineers
Surgical center managers
Databases:
PubMed Orthopedics literature
FDA 510(k)/PMA device database
ClinicalTrials.gov
National joint/implant registries
Magazines:
Podiatry Today
Orthopedics This Week
Becker’s Spine & Orthopedics Review
Footwear News
Journals:
Foot & Ankle International
Journal of Foot & Ankle Surgery
The Journal of Bone & Joint Surgery
Clinical Orthopaedics and Related Research
Newspapers:
The Wall Street Journal (Health)
The Guardian (Science)
Financial Times (Medical Devices)
The Hindu (Health)
Associations:
American Orthopaedic Foot & Ankle Society (AOFAS)
British Orthopaedic Foot & Ankle Society (BOFAS)
International Foot & Ankle Foundation
FDA CDRH
Share
Share
About Author
Manisha Vibhute is a consultant with over 5 years of experience in market research and consulting. With a strong understanding of market dynamics, Manisha assists clients in developing effective market access strategies. She helps medical device companies navigate pricing, reimbursement, and regulatory pathways to ensure successful product launches.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients