Fetal and neonatal monitoring devices are used for monitoring heart rate, blood pressure, oxygen level, and other vital physiological parameters of the fetus and contractions in the mother’s uterus. These devices are used for continuous monitoring of the fetus and mother to avoid any type of harm. These are also used to monitor chronic mental retardation, hypothermia, lung diseases, neonatal diseases, vision & hearing problems, and jaundice in fetus. Fetal monitoring is usually done using handheld Doppler device or an electronic fetal monitor. Fetal and neonatal monitoring devices are continuously evolving in terms of technology. In the recent past, continuous fetal and neonatal monitoring devices were developed, which can be connected with internet and can be accessed from anywhere. Due to these technical developments, fetal and neonatal monitoring market is expected to grow significantly in the near future.
Fetal and Neonatal Monitoring Market - Impact of Coronavirus (Covid-19) Pandemic
The pandemic of COVID-19 has significantly augmented demand for several drugs and devices market. The spread of coronavirus is also positively impacting the sales of fetal and neonatal monitoring products, as the rate of child birth has not reduced. Furthermore, the U.S.FDA eases rules for imaging systems, fetal and maternal monitoring devices amid COVID-19. For instance, in April 2020, the U.S. FDA issued guidance detailing relaxed policies for imaging systems and non-invasive fetal and maternal monitoring devices to increase availability of the devices amid the coronavirus disease (COVID-19) pandemic. Within the guidance, FDA explains that for the duration of the public health emergency, it does not intend to object to certain modifications made to imaging systems and image analysis software, including modifications to make imaging systems mobile or portable.
The global fetal and neonatal monitoring market was valued at US$ 8,101.1 million in 2020 and is expected to exhibit a CAGR of 6.9% over the forecast period (2019-2027).
Figure 1: Global Fetal and Neonatal Monitoring Market Share (%) Analysis, By Product Type, 2019
To learn more about this report, Download Free Sample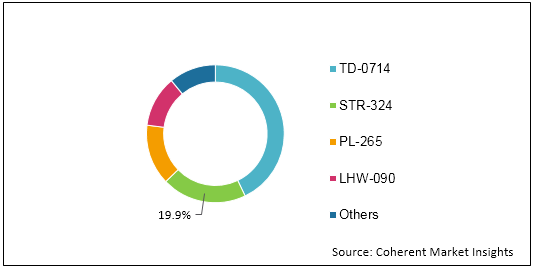
Increasing FDA Approvals and Technological Advancements are expected to Drive Growth of the Fetal and Neonatal Monitoring Market over the Forecast Period.
The fetal and neonatal monitoring market is expected to witness significant growth, owing to increasing number of product approvals by the U.S. FDA over the forecast period. For instance, in December 2019, Masimo announced that RD SET sensors with Masimo Measure-through Motion and Low Perfusion SET pulse oximetry received the U.S. FDA clearance for improved oxygen saturation (SpO2) accuracy specifications for neonatal patients (<3 kg).
Moreover, manufacturers are highly focused on technological developments such as internet-based devices, Bluetooth-based devices, continuous monitoring devices, and others in the fetal and neonatal monitoring systems. Such technological advancements in the fetal and neonatal monitoring systems is expected to boost the fetal and neonatal monitoring market growth over the forecast period. For instance, Novii Wireless Patch System by General Electric Company is a maternal/fetal monitor that non-invasively measures and displays fetal heart rate, maternal heart rate, and uterine activity. The patch incorporates ECG electrode areas, which pick up ECG and EMG signals from the skin surface and then transfers them to the Novii Pod.
Fetal and Neonatal Monitoring Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2019 | Market Size in 2019: | US$ 8,101.1 Mn |
| Historical Data for: | 2016 to 2019 | Forecast Period: | 2020 to 2027 |
| Forecast Period 2020 to 2027 CAGR: | 6.9% | 2027 Value Projection: | US$ 12,960.0Mn |
| Geographies covered (27): |
|
||
| Segments covered: |
|
||
| Companies covered: |
Becton, Dickinson and Company, Fujifilm SonoSite Inc., Medtronic Plc, Dragerwerk AG & Co. KGAA, Cooper Surgical, Getinge AB, Natus Medical Incorporated, GE Healthcare, Siemens Healthcare GmbH, ArjoHuntleigh, Inc., Neoventa Medical, Koninklijke Philips N.V., Analogic Corporation, Spacelabs Healthcare Inc., Smiths Medical, and Masimo. |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Fetal and Neonatal Monitoring Market - Restraints
The major restraining factor for the fetal and neonatal monitoring market include product recalls by the regulatory bodies. The product recalls arise due to product failure, component inconsistency, and packaging problems. For instance, in October 2019, the U.S. FDA recalled the Mindray DS USA, Inc.’s (a subsidiary of Mindray Medical International Limited) Rosie4 Vital Signs Monitor, which is used for monitoring physiologic parameters, including Pulse Oximetry (SpO2), Pulse Rate (PR), Non-invasive Blood Pressure (NIBP), Temperature (TEMP), and Carbon Dioxide (CO2) on adult, pediatric, and neonatal patients in professional healthcare facilities. The product was recalled by the U.S. FDA because the NIBM valve system was not included in the marketing clearance. The company initiated the recall but not all products were corrected or removed.
Fetal and Neonatal Monitoring Market - Regional Insights
North America is expected to hold a dominant position in the global fetal and neonatal monitoring market during the forecast period, owing to increasing R&D activities by research institutes in fetal and neonatal monitoring systems. For instance, in August 2019, the researchers at Stevens Institute of Technology developed a wearable device designed to help expecting parents keep track of their child’s fetal heartbeat. The lightweight technology uses seismo-cardiogram and gyro-cardiogram recordings collected from a consumer-grade sensor. When placed on the mother’s stomach, it detects vibrations, when the baby is moving or its heart is beating.
Moreover, Asia Pacific is expected to witness growth in the fetal and neonatal monitoring market in 2027. The market in the Asia Pacific is expected to gain momentum during the forecast period, owing to an increasing neonatal mortality rate. For instance, according to a study “State of newborn health in India’’ the neonatal mortality rate was 28 per 1,000 live births in India in 2017, which is quite high when compared to western countries. Thus, increasing neonatal mortality rates in Asia Pacific countries are expected to drive demand for fetal and neonatal monitoring devices over the forecast period.
Figure 2: Global Fetal and Neonatal Monitoring Market Value (US$ Mn) & Y-o-Y Growth (%), 2017-2027
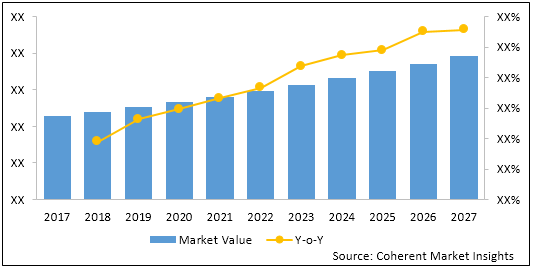
To learn more about this report, Download Free Sample
Fetal and Neonatal Monitoring Market - Competitive Landscape
Key players operating in the global fetal and neonatal monitoring market include Becton, Dickinson and Company, Fujifilm SonoSite Inc., Medtronic Plc, Dragerwerk AG & Co. KGAA, Cooper Surgical, Getinge AB, Natus Medical Incorporated, GE Healthcare, Siemens Healthcare GmbH, ArjoHuntleigh, Inc., Neoventa Medical, Koninklijke Philips N.V., Analogic Corporation, Spacelabs Healthcare Inc., Smiths Medical, and Masimo.
Share
Share
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients