Europe Heparin Market is estimated to be valued at USD 3,915.9 Mn in 2025 and is expected to reach USD 6,045.4 Mn in 2032, exhibiting a compound annual growth rate (CAGR) of 6.4% from 2025 to 2032.
Analysts’ views on Europe Heparin Market :
The Heparin, also known as unfractionated heparin, is a medication and naturally occurring glycosaminoglycan. Since heparins depend on the activity of antithrombin, they are considered anticoagulants. Specifically it is also used in the treatment of heart attacks and unstable angina. Heparin is also used to prevent blood clotting during open-heart surgery, bypass surgery, kidney dialysis, and blood transfusions. It is used in low doses to prevent the formation of blood clots in certain patients, especially those who must have certain types of surgery or who must remain in bed for a long time. Heparin may also be used to diagnose and treat a serious blood condition called disseminated intravascular coagulation.
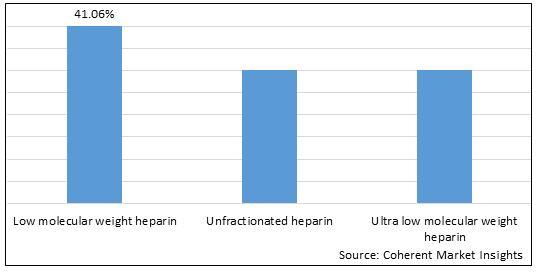
Figure 1. Europe Heparin Market Share (%), by Product Type, 2025
To learn more about this report, Download Free Sample
Europe Heparin Market– Driver
Investment by key players owing the growth of market
To expand markets internationally, Europe companies are focused on investments to expand its product portfolio. For instance, On May 4, 2023, Vall Companys, agri-food group and the Bioiberica,a spain based pharmaceutical comapny have presented Biovall Heparin Science, a joint business project that will enable the production of crude heparin extracted from porcine intestinal mucosa. The project, which has been approved by the local government, is notable for being the union of two corporations in its respective sectors. Maximum traceability from the mucosa to heparin and the utmost product safety and quality.
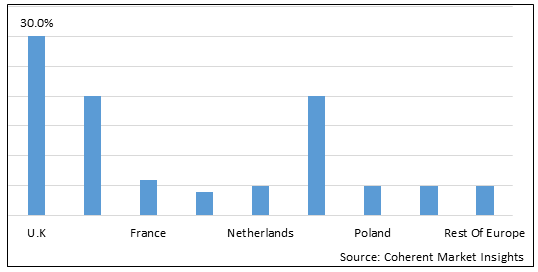
Figure 2. Europe Heparin Market Share (%), by Country, 2025
To learn more about this report, Download Free Sample
Europe Heparin Market- Regional Analysis
Among country, U.K. is the fastest-growing segment in the Europe heparin market over the forecast period. According to an article published in Journal of EuroPCR and the European Association of Percutaneous Coronary Interventions, on August 29, 2025, explained pretreatment with unfractionated heparin was associated with a reduction in coronary artery occlusion among patients with segment elevation myocardial infarction, with a number needed to treat (NNT) of 12, without increasing the risk of major in-hospital bleeding. Regarding mortality, a reduction was found with unfractionated heparin pretreatment, with an NNT of 94, but this effect was not robust over all sensitivity analyses and residual confounding cannot be excluded.
Europe Heparin Market– Impact of Coronavirus (COVID-19) Pandemic
Since the COVID-19 virus outbreak in December 2019, the disease has spread to over 100 countries across the globe, and the World Health Organization declared it a public health emergency on January 30, 2020.
COVID-19 affected the economy in three main ways: by directly affecting the production and demand of drugs, by creating disruptions in distribution channels, and through its financial impact on firms and financial markets. Due to nationwide lockdowns, several countries, such as China, India, Saudi Arabia, the U.A.E., Egypt, and others faced problems regarding transportation of drugs from one place to another.
For instance, on April 27, 2023 an article published by Frontiers explained although the COVID-19’s pathophysiology remains relatively unclear, it is well established that coagulopathy, systemic thrombotic propensity, and a robust immunoinflammatory response are some of the most important determinants of its morbidity and mortality. Accordingly, research efforts have focused on addressing the inflammatory and hematological cascades using available agents to avoid thromboembolic events. Several studies and investigators have emphasized the importance of Low molecular weight heparin (LMWH), namely, Lovenox, in addressing these sequelae of the COVID-19 disease, either prophylactically or therapeutically.
Europe Heparin Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 3,915.9 Mn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 6.4% | 2032 Value Projection: | USD 6,045.4 Mn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Pfizer, Inc., Sagent Pharmaceuticals, Inc., B. Braun Melsungen AG, Baxter International Inc, GlaxoSmithKline plc, Bayer AG, Anselm Pharmaceuticals, Bristol-Myers Squibb Co, Dr. Reddy’s Laboratories Ltd, Fresenius SE & Co. KGaA, Leap Labchem Co, LEO Pharma A/S, Sanofi S.A, Syntex S.A., Teva Pharmaceutical Industries Ltd, United Biotech (P) Ltd, Amphastar Pharmaceuticals Inc., Abbott Laboratories, Aspen Pharmacare Holdings, Laboratorios Farmaceuticos ROVI SA, Intrapharm Laboratories. |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Europe Heparin Market Segmentation:
Europe Heparin Market report is segmented into Product Type, By End User and by country.
Based on Product Type, the Europe heparin market is segmented low molecular weight heparin, unfractionated heparin, and ultra low molecular weight heparin. Out of which the low molecular weight heparin is expected to dominate the market over the forecast period and this is due to ease of availability and growing approval for novel drugs.
Based on End User, the Europe heparin market is segmented into Hospitals, Blood and Stem Cell Bank, and Others (Laboratories, Acute Care Centers). The hospital segment is expected to dominate the market over the forecast period and this is due to the increasing number of hospitalizations.
Based on Country, the Europe heparin market is segmented into Germany, France, the U.K., Italy, Spain, Russia, The Netherlands, Poland, Switzerland, Sweden, and the Rest of Europe. The U.K segment is expected to dominate the market over the forecast period and this is due to leading pharmaceutical and biotechnological companies having a strong presence in the region.
Among all segmentation, the product type segment has the highest potential as increasing research and development in heparin market . For instance, on October 6, 2022, The Royal Society of Chemistry, U.K based journal stated that low molecular weight heparins could provide a targeting capability to nanoparticle through interactions with extracellular enzymes or cell membrane biomarkers, as well as provide an antitumour activity.
Europe Heparin Market Cross Sectional Analysis:
Among the product type segmentation, the low molecular weight heparin segment has maximum potential in Germany owing to the rising research and development. For instance, on October 11, 2022, according to an article published by Georg Thieme Verlag KG, Europe based journal explained the supply of heparins is to disseminate the use of products from alternative animal sources, such as bovine intestine mucosa (HBI), which currently accounts for only 1% of the unfractionated heparin consumed worldwide, and ovine intestine mucosa (HOI) in development as new unfractionated heparin and low-molecular-weight heparin products.
Europe Heparin Market: Key Developments
In 2022, Cardiff University, a research university development of drug delivery systems that release cancer drugs in a controlled manner and a sustainable way is still difficult. Hypothesis that highly sulfated heparin-based microcarriers would enable electrostatic binding and controlled drug release. In silica modeling showed that the cancer drug doxorubicin has an affinity for the heparin component of microcarriers. Experiments have shown the existence of a strong electrostatic interaction reversible, allowing both loading and subsequent slow release of doxorubicin over 42 days without letting go of the first explosion. The drug-loaded microcarriers were able to reduce cancer cells viability in vitro in both hormone-dependent and highly aggressive tri-negative individuals breast cancer cells.
On May 27, 2023, European Bioinformatics Institute, explained fibroblast growth factors are heparin-binding proteins, and interactions with cell surface-bound heparan sulfate proteoglycans have been shown to be important for FGF signaling. FGFs have an intrinsic pseudo-threefold symmetry (β-trefoil topology). Currently, more than 20 different members of the FGF family have been identified in mammals, all of which are structurally similar signaling molecules. They exert its effects through four different membrane fibroblast growth factor receptors (FGFRs), FGFR1 to FGFR4, which belong to the tyrosine kinase superfamily. Upon binding to FGF, the receptors dimerize and its intracellular tyrosine kinase domains are activated.
Europe Heparin Market: Key Trends
Increasing usage and application of heparin
Increasing usage and application of heparin can drive the growth of Europe Heparin Market. On March 1, 2023, Life science regulation in spain, explained medical devices incorporating a medicinal product in complementary manner are exclusively subject to the medical device regime if the medicinal product only has an effect in the human body complementary to the effect exerted by the medical device for example, a bone cement with antibiotics, catheters with heparins. This also applies if the medical device only incorporates a component of the medicinal product or a medicinal product derived from human blood or plasma. Heparin in catheters is a innovative techquie.
Europe Heparin Market: Restraint
Risk of occurrence of diseases due to heparin
According to EuropPCR, based in Paris, on September 1, 2022, explained crude models showed that pretreatment with unfractionated heparin was associated with a lower risk of coronary occlusion on angiography, 30-day mortality, and major in-hospital bleeding compared with no pretreatment. Regardless of the number of covariates included in the model, the derived multivariate Poisson regression showed consistent results for coronary occlusion in angiography. However, adjusted risk estimates for in-hospital major bleeding were not significant.
To counterbalance this restraint, a robust protocol should be introduce to develop pure form of hepain drug.
Europe Heparin Market - Key Players
Major players operating in the Europe heparin market include Pfizer, Inc., Sagent Pharmaceuticals, Inc., B. Braun Melsungen AG, Baxter International Inc, GlaxoSmithKline plc, Bayer AG, Anselm Pharmaceuticals, Bristol-Myers Squibb Co, Dr. Reddy’s Laboratories Ltd, Fresenius SE & Co. KGaA, Leap Labchem Co, LEO Pharma A/S, Sanofi S.A, Syntex S.A., Teva Pharmaceutical Industries Ltd, United Biotech (P) Ltd, Amphastar Pharmaceuticals Inc., Abbott Laboratories, Aspen Pharmacare Holdings, Laboratorios Farmaceuticos ROVI SA, Intrapharm Laboratories.
*Insight: Heparin injection is an anticoagulant. It is used to decrease the clotting ability of the blood and help prevent harmful clots from forming in blood vessels. This medicine is sometimes called a blood thinner, although it does not actually thin the blood.
Share
Share
About Author
Ghanshyam Shrivastava - With over 20 years of experience in the management consulting and research, Ghanshyam Shrivastava serves as a Principal Consultant, bringing extensive expertise in biologics and biosimilars. His primary expertise lies in areas such as market entry and expansion strategy, competitive intelligence, and strategic transformation across diversified portfolio of various drugs used for different therapeutic category and APIs. He excels at identifying key challenges faced by clients and providing robust solutions to enhance their strategic decision-making capabilities. His comprehensive understanding of the market ensures valuable contributions to research reports and business decisions.
Ghanshyam is a sought-after speaker at industry conferences and contributes to various publications on pharma industry.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients