Epilepsy is a chronic non-communicable disease of the brain that affects people of all ages. Epilepsy can start at any age, but usually starts either in childhood or in people over 60. Seizures can affect people in different ways, depending on which part of the brain is involved. Possible symptoms of epilepsy include uncontrollable jerking and shaking, called ‘fit’, loss of awareness and staring blankly into space, stiffness, strange sensations in the stomach, unusual smell or taste, tingling sensation in arms or legs, and collapsing. The most commonly used treatment methods of epileptic seizures include anti-epileptic drug therapy, brain surgery, vagus nerve stimulation, deep brain stimulation, and ketogenic diet.
Epileptic Seizures Treatment Market – Impact of Coronavirus (Covid-19) Pandemic
The demand for epileptic seizures treatment remained same during COVID-19 pandemic, as it is observed that epilepsy and COVID-19 infection does not have a direct connection. According to the International League against Epilepsy, there is no direct evidence that the coronavirus infection can directly cause epilepsy. However, like most of the infections can cause high fever, breathing difficulties, and other problems with normal functioning, being infected can result in a person who is susceptible to epilepsy, might suffer breakthrough episodes. Although, disruption in the supply of drugs have slightly impacted the epileptic seizures treatment market negatively.
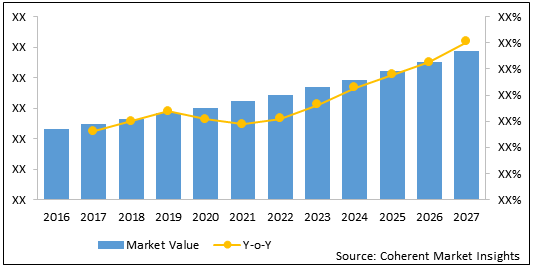
The epileptic seizures treatment market is estimated to be valued at US$ 3.0 billion in 2020 and is expected to exhibit a CAGR of 5.4% during the forecast period (2020-2027).
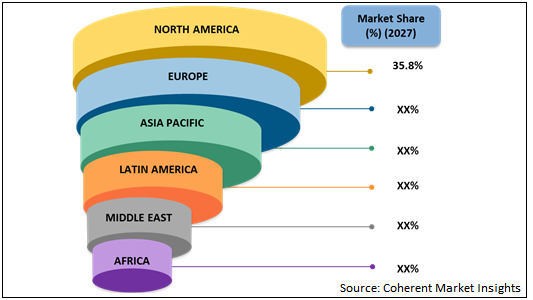
Figure 1. Global Epileptic Seizures Treatment Market Share (%) Analysis, By Region, 2027
To learn more about this report, Download Free Sample
Rising clinical trials and increasing drug approvals & launches are expected to boost growth of the epileptic seizures treatment market
Rising clinical trials for new drug for the treatment of epileptic seizures is expected to accelerate the epileptic seizures treatment market growth over the forecast period. For instance, in January 2019, Xenon Pharmaceuticals Inc. in collaboration with NCGS, Inc. initiated a clinical study on the drug, XEN1101 for the treatment of focal epilepsy. The trial aims to evaluate the clinical efficacy, safety, and tolerability of XEN1101 administered as adjunctive treatment in adult patients aged 18 to 75 years diagnosed with focal epilepsy. In this trial, 300 patients are involved, who are being treated with XEN1101 and placebo capsules. This clinical trial is in phase 2 and is expected to complete in June 2022.
Furthermore, increasing drug approvals and launches for the treatment of epileptic seizures is expected to drive the global epileptic seizures treatment market growth. For instance, Eisai Co., Ltd. announced that they received a New Drug Approval for its in-house discovered and developed anti-epileptic drug (AED) Fycompa (perampanel) from the China National Medical Products Administration (NMPA) for use in an adjunctive treatment of partial onset seizures (with or without secondarily generalized seizures) in epilepsy patients 12 years of age and older. Hence, such approvals are expected to positively impact the global epileptic seizures treatment market growth.
Epileptic Seizures Treatment Market – Restraints
Anti-epileptic drugs exhibit mild or adverse neurological side effects occasionally, however they can be fatal. The adverse reactions and risks vary with each medication. Majority of antiepileptic drugs have the potential to cause an allergic reaction. These drugs indirectly increase the risk of conditions such as aggressive behavior and depression. In pregnant women, anti-epileptic drugs can also harm the fetus. According to the Journal of Family Practice the side effects of anti-epileptic drugs include – Phenytoin causes a pattern of defects collectively called the Fetal Hydantoin Syndrome (FHS) characterized by variable degrees of hypoplasia and ossification of the distal phalanges and craniofacial abnormalities. Moreover, anticonvulsants, particularly hydantoins and barbiturates are associated with hemorrhagic diseases in newborns. Therefore, such severe side effects are expected to hinder the epileptic seizures treatment market growth over the forecast period.
Epileptic Seizures Treatment Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2019 | Market Size in 2020: | US$ 3.0 Bn |
| Historical Data for: | 2017 to 2019 | Forecast Period: | 2020 to 2027 |
| Forecast Period 2020 to 2027 CAGR: |
5.4% |
2027 Value Projection: | US$ 4.5 Bn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Novartis AG, GlaxoSmithKline Plc, Johnson & Johnson Service, Inc., Teva Pharmaceutical Industries Ltd., Pfizer, Inc., Sanofi S.A., Mylan N.V., Bausch Health Companies Inc., UCB S.A., Eisai Co. Ltd., Abbott Laboratories, Sumitomo Dainippon Pharma Co., Ltd., GW Pharmaceuticals Plc, Marinus Pharmaceuticals, Inc., H. Lundbeck A/S, and Takeda Pharmaceutical Co., Ltd. |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Epileptic Seizures Treatment Market – Regional Analysis
North America is estimated to hold dominant position in the global epileptic seizures treatment market in 2020 due to increasing product launches and product approvals by regulatory authorities in the region. For instance, in November 2019, SK Life Science, Inc. announced that the U.S. Food and Drug Administration (FDA) approved XCOPRI (cenobamate tablets) for the treatment of partial-onset seizures in adults. Similarly, in August 2019, Dr. Reddy's Laboratories launched generic anti-epileptic Vigabatrin powder for oral solution in the U.S. market.
Moreover, Asia Pacific epileptic seizures treatment market is expected to witness significant growth during the forecast period, owing to increasing agreements between the key players for supply of anti-epileptic drugs in Asia Pacific region. For instance, in December 2017, Hydroponics Company entered into agreement with BOL Pharma to supply BOL Pharma’s medicinal cannabis products in Australia. BOL Pharma’s medicinal cannabis products are intended to be used for the treatment of patients suffering from epilepsy and other neurological disorders.
Figure 2. Global Epileptic Seizures Treatment Market Value (US$ Bn) & Y-o-Y Growth (%), 2016-2027
To learn more about this report, Download Free Sample
Epileptic Seizures Treatment Market - Competitive Landscape
Key players operating in the global epileptic seizures treatment market include Novartis AG, GlaxoSmithKline Plc, Johnson & Johnson Service, Inc., Teva Pharmaceutical Industries Ltd., Pfizer, Inc., Sanofi S.A., Mylan N.V., Bausch Health Companies Inc., UCB S.A., Eisai Co. Ltd., Abbott Laboratories, Sumitomo Dainippon Pharma Co., Ltd., GW Pharmaceuticals Plc, Marinus Pharmaceuticals, Inc., H. Lundbeck A/S, and Takeda Pharmaceutical Co., Ltd.
Share
Share
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients