Ependymoma is a type of tumor that can form in the brain or spinal cord. Ependymoma rises from the ependymal cells that line the ventricles of the brain and the center of the spinal cord. Ependymoma can occur at any age, but most often occurs in children under 5 years of age . Children with ependymoma may experience headaches and seizures.
Global ependymoma drug market is estimated to be valued at US$ 143.0 million in 2022 and is expected to exhibit a CAGR of 4.8%during the forecast period (2022-2030).
Figure 1.Global Ependymoma Drug Market Share (%) in Terms of Value, By Drug Type, 2022
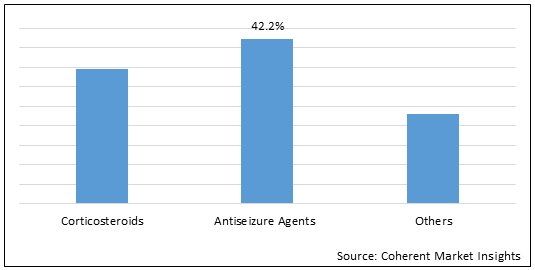
To learn more about this report, Download Free Sample
Increasing prevalence of ependymoma is expected to drive the market growth during the forecast period.
Increasing prevalence of ependymoma is expected to drive the global ependymoma drug market growth over the forecast period. For instance, according to data published by American Society of Clinical Oncology in January 2022, ependymoma occurs most often in young children, accounting for about 5% of all childhood brain cancers. Approximately 250 children and teens under the age of 19 in the U.S. would be diagnosed with ependymoma in 2022.
Ependymoma Drug Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2021 | Market Size in 2022: | US$ 143.0 Mn |
| Historical Data for: | 2017-2020 | Forecast Period: | 2022 to 2030 |
| Forecast Period 2022 to 2030 CAGR: | 4.8% | 2030 Value Projection: | US$ 208.4 Mn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Fera Pharmaceuticals, Pfizer, Novartis AG, Merck KGaA, Baxter, Cipla Limited, Zydus Cadila, Lupin Pharmaceuticals, Inc., UCB, Inc., APOTEX INC, and Moleculin Biotech, Inc. |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Figure 2.Global Ependymoma Drug Market Share (%), By Distribution Channel, 2022
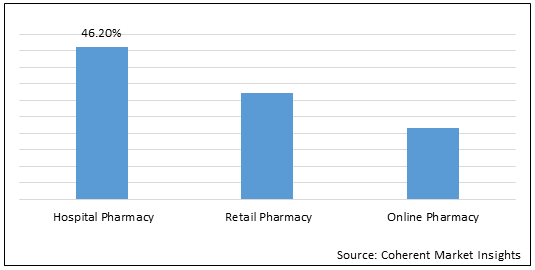
To learn more about this report, Download Free Sample
Increasing product approvals for the treatment of ependymoma from regulatory authorities are expected to drive the market growth during the forecast period
Increasing product approvals for the treatment of ependymoma which is expected to drive the global ependymoma drug market growth during the forecast period. For instance, in April 2021, Moleculin Biotech, Inc., a clinical stage pharmaceutical company, announced that the U.S. Food and Drug Administration (FDA) had granted Rare Pediatric Disease Designation (RPD) to its p-STAT3 inhibitor, WP1066, for the treatment of ependymoma.
Global Ependymoma Drug Market– Impact of Coronavirus (COVID-19) Pandemic
The supply of raw materials required for production of pharmaceuticals has been severely disrupted due to the forced quarantine and lack of labor during the COVID-19 pandemic. As the link between regional warehouses is not smooth, transportation of raw materials between regions cannot be carried out successfully. This shortage of raw materials and components has negatively affected the supply chain of the global ependymoma drug market. Thus, the COVID-19 pandemic is expected to have a negative impact on supply chain of ependymoma drug market. According to an article published by the Exploratory Research in Clinical and Social Pharmacy journal in June 2021, a study was carried out to evaluate the impact of COVID-19 on pharmaceutical systems and supply chain in resource-limited countries in Sub-Saharan countries such as Namibia. This study revealed negative impact on availability and access of essential drugs, sanitation and hygiene products, and antimicrobials. Most pharmaceutical companies and pharmacies in Namibia experienced delayed manufacturing and distribution of drugs, which is attributed to reduced inter-country transportation of pharmaceutical goods and limited in-country capacity to manufacture drugs. For instance, according to a review article published by the European Pharmaceutical Review Journal in November 2020, COVID-19 pandemic has caused major challenges with respect to drug shortages and increased manufacturing costs across the globe. Moreover, the same source stated that COVID-19 pandemic has also led to problems such as stockpiling drugs, transportation delay, and others. Some measures that can be taken to ease the supply of pharmaceuticals during the pandemic include next generation technologies such as digital network platforms designed to work across multiple pharmaceutical enterprises and ensure the timely delivery of drugs to patients across the globe.
Global Ependymoma Drug Market: Restraint
The major factors that hinder growth of the global ependymoma drug market include side effects associated with the medication. Dexamethasone a corticosteroid used in the treatment of ependymoma causes side effects which include upset stomach, stomach irritation, vomiting, headache, dizziness, insomnia, restlessness, depression, anxiety, acne, increased hair growth, easy bruising etc.
Key Players
Key players operating in market include Fera Pharmaceuticals, Pfizer, Novartis AG, Merck KGaA, Baxter, Cipla Limited, Zydus Cadila, Lupin Pharmaceuticals, Inc., UCB, Inc., APOTEX INC, and Moleculin Biotech, Inc.
Share
Share
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients