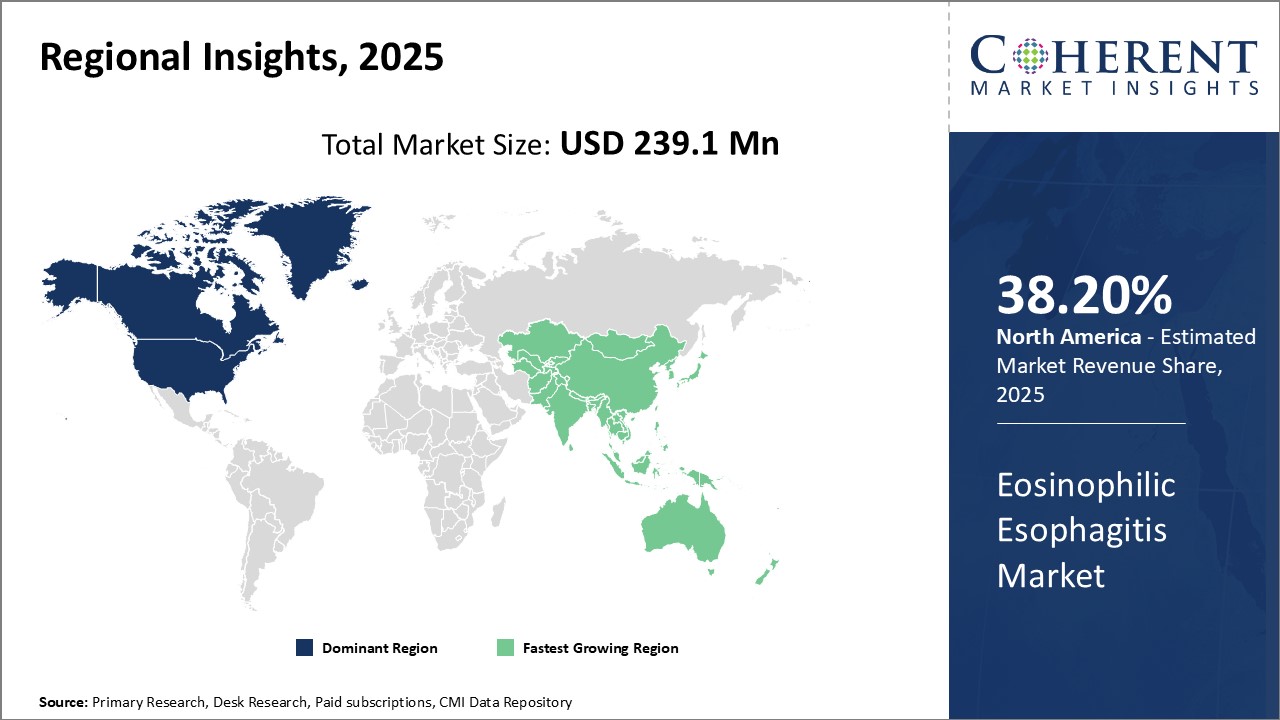
Global eosinophilic esophagitis market is estimated to be valued at USD 239.1 Mn in 2025 and is expected to exhibit a CAGR of 33.4% during the forecast period (2025-2032). Eosinophilic esophagitis (EoE) is a chronic immune system disease of the esophagus. In EoE, a large number of white blood cells called eosinophils are found in the inner lining of the esophagus. This buildup, which is a reaction to food, allergen, or acid reflux, can inflame or injure the esophageal tissue. Difficulties in swallowing, impaction, chest pain often centrally located that does not respond to antacids, and the backflow of undigested food are the signs and symptoms of eosinophilic esophagitis in adults.
Figure 1. Global Eosinophilic Esophagitis Market Value (USD Mn), by Region, 2025
To learn more about this report, Download Free Sample
Promising results for potential drug candidates are estimated to drive the global eosinophilic esophagitis market growth during the forecast period.
Eosinophilic Esophagitis Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 239.1 Mn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 33.4% | 2032 Value Projection: | USD 1,797.4 Mn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Ellodi Pharmaceuticals, EsoCap AG, GlaxoSmithKline plc., Teva Pharmaceutical Industries Ltd., Cipla Limited, Sun Pharmaceutical Industries Limited, AstraZeneca Plc, Sanofi S.A., Arena Pharmaceuticals, Inc., Takeda Pharmaceutical Company Limited, Revolo Biotherapeutics, Allakos Inc., Bristol-Myers Squibb Co, Calypso Biotech, DBV Technologies, Landos Biopharma, Inc., Glenmark Pharmaceuticals, Alkem Laboratories Ltd., Quorum Innovations LLC, and Dr. Falk Pharma GmbH |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
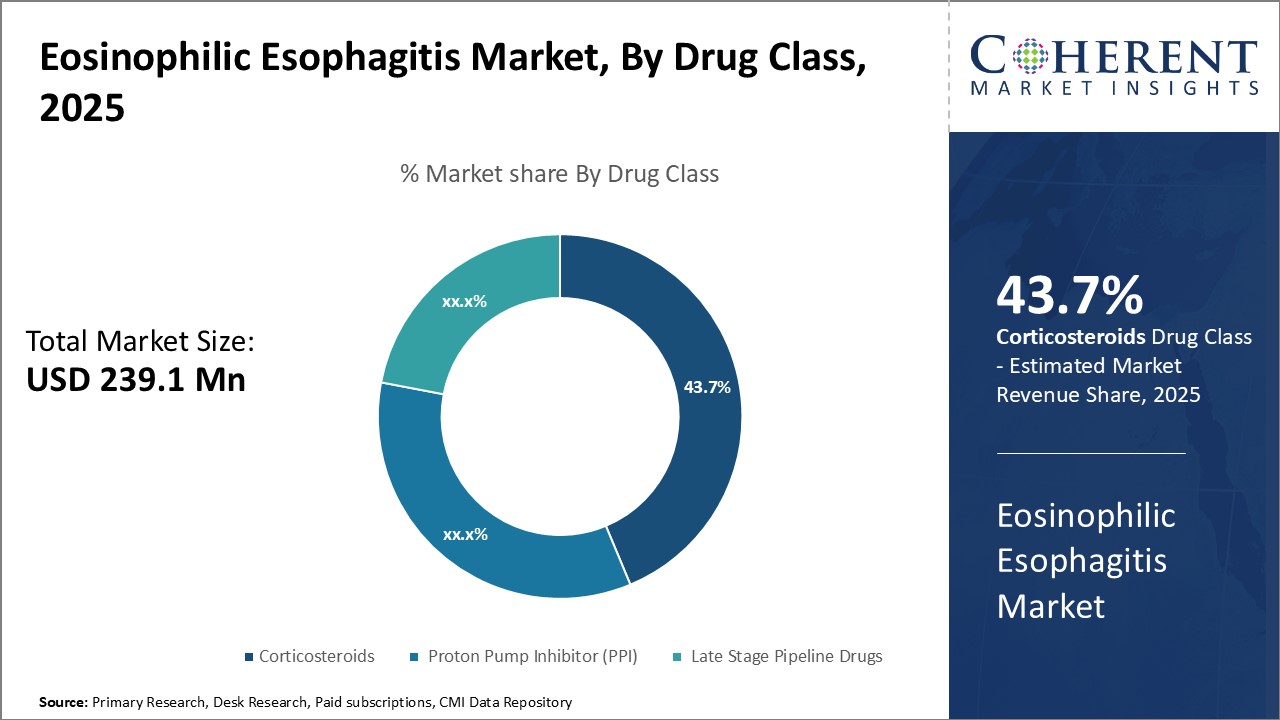
Figure 2. Global Eosinophilic Esophagitis Market Share, By Drug Class, 2025
To learn more about this report, Download Free Sample
Recent Developments
On February 12, 2024, Takeda Pharmaceutical Company Limited, a multinational pharmaceutical company, announced that the U.S. Food and Drug Administration has approved Eohilia (budesonide oral suspension) for the treatment of eosinophilic esophagitis (EoE) in patients aged 11 years and older. The regulatory move makes Eohilia the first and only approved oral medication for this patient population, and Takeda Pharmaceutical Company Limited expects Eohilia 2 mg/10 mL single-dose stick packs to be available by the end of February 2024.
On January 30, 2024, Revolo Biotherapeutics, a biotechnology company, announced that the U.S. Food and Drug Administration (FDA) has given Orphan Drug Designation (ODD) to '1104, a first-in-class immune-resetting peptide being researched as a potential treatment for eosinophilic esophagitis. Revolo submitted its initial request prior to the start of its Phase 2a EoE trial (RVLO 121-04) and revised it following the positive results of an additional Phase 2 study.
On January 25, 2024, Regeneron Pharmaceuticals, Inc., a leading biotechnology company, and Sanofi, a pharmaceutical and healthcare company, announced that the U.S. FDA has approved Dupixent (dupilumab) for the treatment of eosinophilic esophagitis (EoE) in pediatric patients aged 1 to 11 years weighing at least 15 kg. Dupixent is presently the first and only drug approved in the U.S. exclusively for treating these patients.
On January 8, 2024, Calypso Biotech BV, a leader in the development of Interleukin-15 (IL-15) targeted therapies, announced that it has entered into an agreement to be acquired by Novartis AG, a pharmaceutical company. Novartis AG now owns complete rights to CALY-002. Novartis AG plans to further investigate CALY-002 in a wide range of autoimmune diseases with significant unmet medical needs. CALY-002 is now being tested in a Phase 1b trial for patients with Celiac Disease and Eosinophilic Esophagitis.
Increasing awareness programs for Eosinophil-associated disorders is anticipated to propel the global eosinophilic esophagitis market growth over the forecast period.
Various awareness programs have been developed and implemented to educate the public and medical community about eosinophil-associated disorders which is anticipated to drive the market growth over the forecast period. The American Partnership for Eosinophilic Disorders (APFED), a nonprofit organization, was founded in December 2001, which works on educating, raising awareness, supporting, and advocating for patients and families who are suffering from eosinophil-associated diseases.
Global Eosinophilic Esophagitis Market – Impact of Coronavirus (COVID-19) Pandemic
Global Eosinophilic Esophagitis Market: Restraints
As of now, no specific treatment has been approved for eosinophilic esophagitis in the U.S. Market players are focusing on developing drug candidates for the treatment of EoE. However, there are many challenges associated with obtaining approval from the regulatory authorities which is anticipated to hinder the market growth over the forecast period. Obtaining approval for the drug from regulatory authorities is a tedious and challenging as regulatory authorities such as the U.S. Food and Drug Administration (FDA) impose substantial and burdensome requirements upon companies involved in the clinical development, manufacturing, marketing, and distribution of drugs
Key Players
Major players operating in the global eosinophilic esophagitis market include Ellodi Pharmaceuticals, EsoCap AG, GlaxoSmithKline plc., Teva Pharmaceutical Industries Ltd., Cipla Limited, Sun Pharmaceutical Industries Limited, AstraZeneca Plc, Sanofi S.A., Arena Pharmaceuticals, Inc., Takeda Pharmaceutical Company Limited, Revolo Biotherapeutics, Allakos Inc., Bristol-Myers Squibb Co, Calypso Biotech, DBV Technologies, Landos Biopharma, Inc., Glenmark Pharmaceuticals, Alkem Laboratories Ltd., Quorum Innovations LLC, and Dr. Falk Pharma GmbH.
Share
Share
About Author
Ghanshyam Shrivastava - With over 20 years of experience in the management consulting and research, Ghanshyam Shrivastava serves as a Principal Consultant, bringing extensive expertise in biologics and biosimilars. His primary expertise lies in areas such as market entry and expansion strategy, competitive intelligence, and strategic transformation across diversified portfolio of various drugs used for different therapeutic category and APIs. He excels at identifying key challenges faced by clients and providing robust solutions to enhance their strategic decision-making capabilities. His comprehensive understanding of the market ensures valuable contributions to research reports and business decisions.
Ghanshyam is a sought-after speaker at industry conferences and contributes to various publications on pharma industry.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients