Enteral Syringe Market
Enteral syringes are majorly used for administering drugs and nutritional therapy through oral, rectal or sublingual route of administration. They are commonly used in hospitals, clinics, and homes and are available in range of 1ml to 60 ml. Newly launched enteral syringes are designed and manufactured to meet latest international standard ISO-80369-3. Drugs can be directly placed into the gastro-intestinal tract through enteral route. Enteral syringes are most widely used for delivering oral liquid medication and nutrition. These syringes works like enteral feedings in its function and end use. Enteral syringes come with purple plunger, which indicates that that the medication in the syringe must be administered via enteral route. Purple color is used in order to separate these syringes from IV syringes. These syringes are utilized for various purpose such as correct measurement of liquid medicine to be administered to patient in situation where dose cannot be accurately measured using a medicine cup or spoon, for administering medicine to infants and young children, and administering controlled drug liquid medicines. These syringes are available in different types such as single use syringes and home use syringes.
The global enteral syringe market size was valued at US$ 575.6 million in 2017 and is expected to witness a CAGR of 4.9% over the forecast period (2018 – 2026).
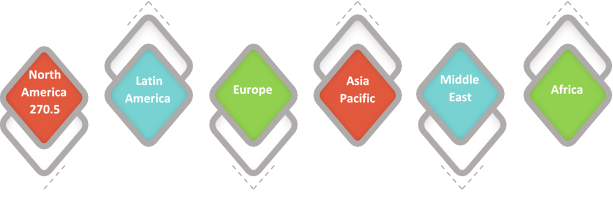
Figure 1. Global Enteral Syringe Market Value (US$ Mn), by Region, 2017
To learn more about this report, Download Free Sample
Source – Coherent Market Insights Report
Increasing number of critical care and neonatal intensive care unit admissions and increasing geriatric population are expected to drive growth of the global enteral syringe market.
Increasing number of critical care and neonatal intensive care unit admissions is expected to drive growth of the global enteral syringes market. This is owing to inability of patients admitted to critical care and newborns admitted to neonatal intensive care (due to premature birth or other complications) to swallow medicine or take nutrition. According to statistics given by Bliss Organization, over 95,000 newborns are admitted to neonatal units in the U.K, annually due to premature birth (before 37 weeks of pregnancy) or due to full term (after 37 weeks) yet sick birth. Moreover, according to same source around 1 in every 8 newborns in the U.K are admitted to neonatal units, annually.
Furthermore, increasing geriatric population is expected to boost the market growth over the forecast period. People above 60 years of age are more prone to illness or injuries that often require hospital admissions and nutritional therapy and medication administration via enteral syringe. According to United Nations report: World Population Prospect-The 2017 Revision, worldwide geriatric population is expected to increase from 962 million in 2017 (13% of worldwide population) to 1.3 billion by 2030 and will reach to 2.1 billion by 2050. According to same source, worldwide geriatric population is increasing at a rate of 3%, annually and around all regions, except Africa, are expected to register geriatric population of over 25% by 2050.
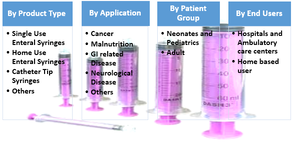
Figure 2. Global Enteral Syringe Market, Segmentation, 2017
To learn more about this report, Download Free Sample
Source – Coherent Market Insights Report
Updated regulatory guidelines for enhanced safety features associated with enteral syringe are expected to propel growth of the market
Regulatory guidelines for design and manufacturing of enteral devices including enteral syringe were recently updated by various regulatory bodies. In 2015, new design standards were introduced in enteral feeding sets, enteral syringes, and enteral feeding tubes referred as ENFit. ENFit is a new connection standard that is developed by an international group of clinicians, manufacturers body Global Enteral Device Supplier Association (GEDSA), and regulators under aegis of Stay Connected initiative to prevent tubing misconnections and patient injury. Tubing misconnection occurs when enteral devices are connected to non-enteral devices such as IV lines, urinary catheters, and ventilator tubing. Global Enteral Device Supplier Association (GEDSA) promotes safe use of enteral devices including syringes. Market players that comply with ENFit standard design are receiving 510(k) clearance from the U.S Food and Drug Administration (U.S. FDA). For instance, in 2016, NeoMed and Medtronic received the U.S FDA 510(k) clearance for low dose tip ENFit syringes. NeoMed has offered royalty-free access of its design to all syringe manufacturers. Therefore, several manufacturers can implement this design standard to promote safe use of these devices, which in turn is expected to increase adoption of enteral syringes.
Key players operating in the global enteral syringe market include, B. Braun Melsungen AG, GBUK Enteral Ltd., Thermo Fischer, Inc., Koninklijke Philips N.V., Miktell Ltd., Baxter International, Inc., Cardinal Health, Inc., Terumo Corporation, Kentec Medical, Inc., Vygon S.A., and others.
Share
Share
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients