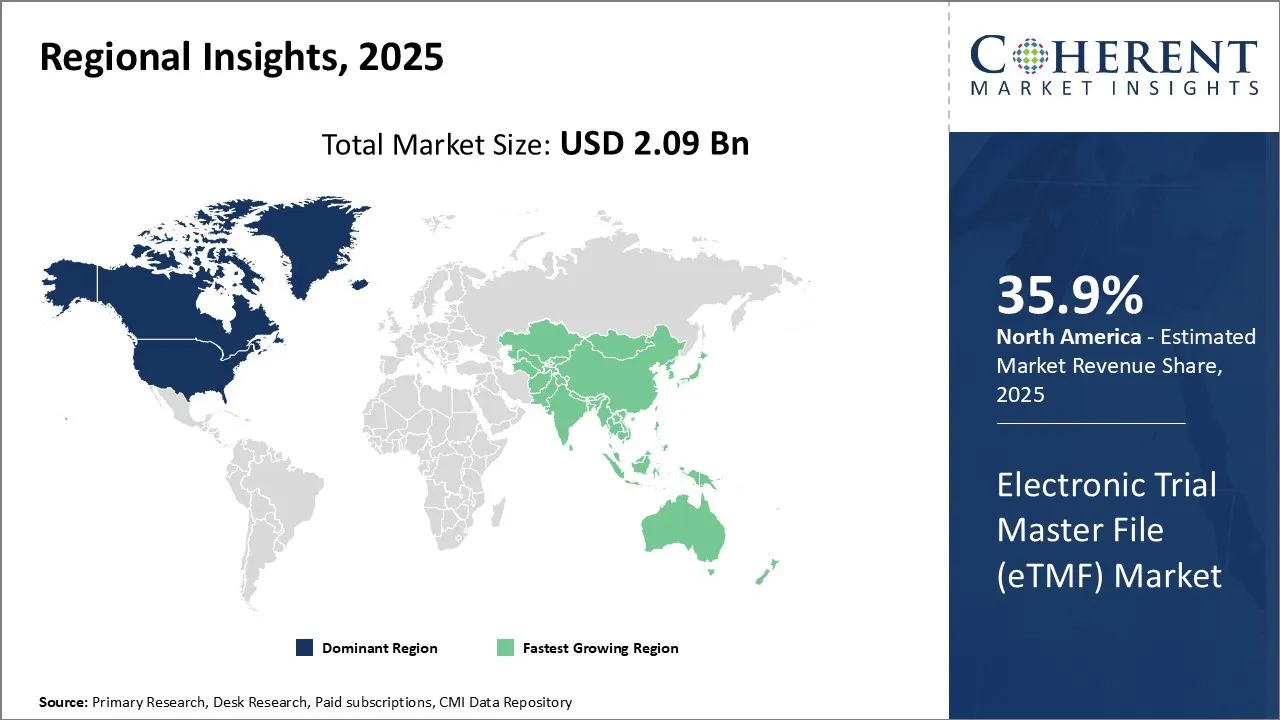
The Electronic Trial Master File (eTMF) market is estimated to be valued at USD 2.09 Bn in 2025 and is expected to reach USD 4.81 Bn by 2032, exhibiting a compound annual growth rate (CAGR) of 12.6% from 2025 to 2032.
To learn more about this report, Download Free Sample
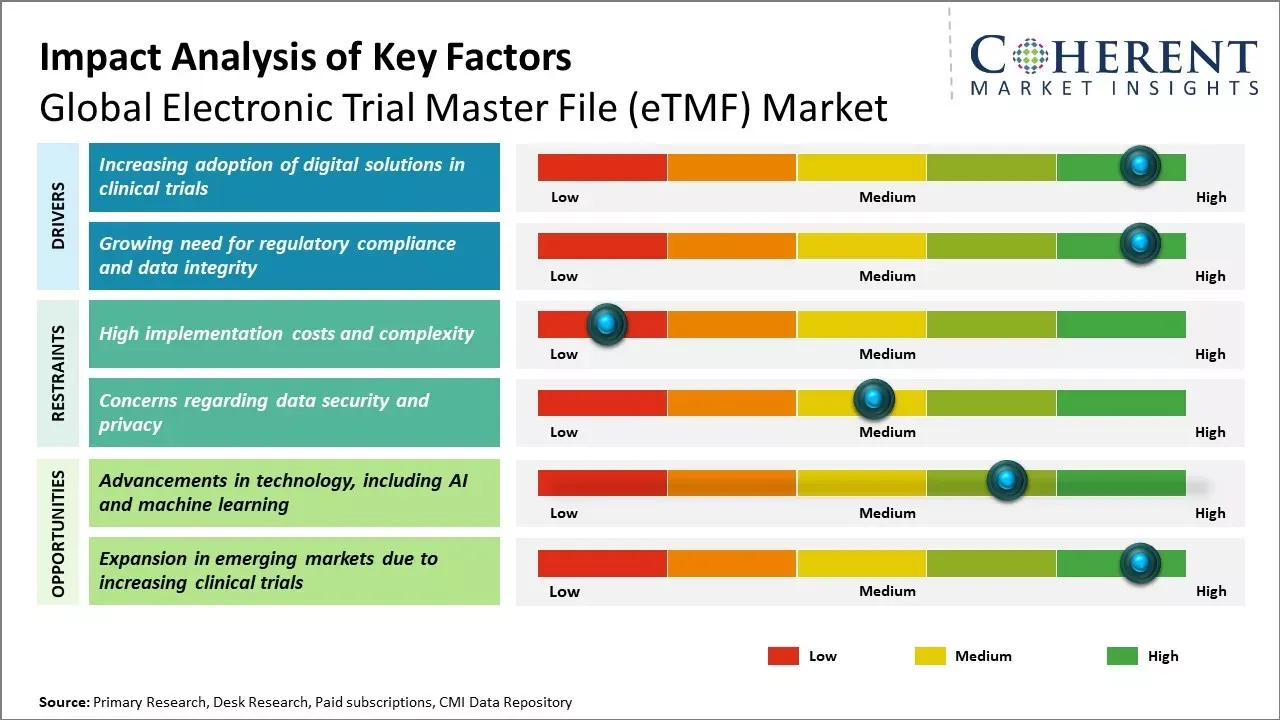
With regulatory compliance becoming increasingly important in clinical trials, there has been huge demand for electronic systems that facilitate regulatory submission and audit preparation. These systems make document management and information retrieval more efficient across global clinical trial sites due to rising adoption of digitalization among pharmaceutical and clinical research organizations.
However, global electronic trial master file (eTMF) market growth can be hampered due to high implementation costs and complexity.
To learn more about this report, Download Free Sample
|
Current Events |
Description and its impact |
|
Regulatory Modernization Initiatives |
|
|
Technological Disruptions |
|
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Pharmaceutical and life sciences industry has undergone tremendous transformation due to advancement of digital technologies. Clinical trials which were traditionally carried out are now transitioning towards digital solutions. There has been risk of important documents being misplaced or lost which could potentially jeopardize patient safety as well delay development timelines.
For instance, as per a study by the Applied Clinincal Trials, in June 2025, USD 1.05 million was invested in AI/ML use by activity assessed, teams experienced an average time reduction of 18% using AI/ML, and overall, respondents reported a positive outlook on the use of AI/ML in drug development.
Managing clinical trials in adherence to rigorous regulatory standards and maintaining the highest level of data integrity is crucial in drug development process. Documents could be misplaced, data transcription errors might occur while transferring information between paper to systems. Regulatory audits involving huge volumes of paper files are challenging and delays in document retrieval impacted audit outcomes. With globalization of clinical trials, demonstrating compliance to authorities across country-specific regulations was challenging with paperwork.
In terms of deployment mode, the cloud-based segment is estimated to contribute the highest market share of 62.6% in 2025, owing to its flexibility and scalability.
Cloud-based eTMF solutions allow life sciences organizations, especially smaller companies and CROs, to avoid large upfront infrastructure costs associated with on-premise implementations. These pay an annual or monthly subscription fee for cloud-hosted services. This capital expenditure model makes eTMF technology more accessible for cost-sensitive customers.
In terms of functionality, the document management segment is estimated to contribute the highest market share of 40.8% in 2025, due to the need for standardized, compliant processes around document control.
Clinical trials and product development projects involve numerous documents whose integrity and traceability must be assured according to regulatory standards. Paper-based systems are prone to errors of document misfiling, lost revisions, and inconsistent naming conventions that undermine data quality.
In terms of end user, the pharmaceutical companies segment is estimated to contribute the highest market share of 49% in 2025, owing to stringent regulations within the industry.
Global pharmaceutical manufacturers face intense scrutiny from public health authorities to demonstrate safety and efficacy of new products. Computerized systems that assure proper conduct of clinical trials and regulatory compliance have therefore become business-critical for these organizations.
Application: The application of machine learning and artificial intelligence (AI) in eTMF has grown significantly. These technologies have been used more and more to automate repetitive processes including document redaction, metadata tagging, and classification. Faster document processing, less human errors, and more overall clinical trial document management efficiency are the outcomes of this automation.
Example: In November 2024, Veeva Systems announced Vault CRM Bot and Vault CRM voice control, two new GenAI capabilities in Vault CRM. Unveiled at Veeva Commercial Summit Europe, CRM Bot and Voice Control join a host of new innovations coming to Vault CRM next year. With these new capabilities, companies can deploy AI that will have immediate value by boosting field productivity.
Application: Another emerging innovation is the use of Blockchain technology to create immutable, time-stamped audit trails within eTMF systems. Regulatory agencies like the U.S. Food and Drug Administration (FDA) emphasize the importance of traceability and unaltered data. Blockchain allows every modification to a document or metadata field to be permanently recorded and time-stamped, making tampering nearly impossible. This is especially crucial in decentralized clinical trials where multiple stakeholders access and modify trial documents across locations.
Example: Startups and R&D teams are piloting Blockchain-enabled eTMF systems where each transaction whether a document upload, review, or approval is securely recorded in a distributed ledger. Companies like Triall are exploring this to enhance transparency and regulatory trust in multi-site and remote clinical trials.
Electronic Trial Master File (eTMF) management in clinical trials is being revolutionized by AI-powered solutions.
To learn more about this report, Download Free Sample
North America has firmly established itself as the dominant region in the global electronic trial master file (eTMF) market with an estimated market share of 35.9% in 2025. With well-developed healthcare infrastructure and stringent regulations regarding clinical trials, the U.S. and Canada have emerged as key hubs for pharmaceutical R&D activities.
For instance, as per an article published by Novotech CRO, it is estimated that by 2025, artificial intelligence will manage 50% of trial data tasks, cutting timelines by 20% and improving data precision. This AI-driven efficiency will enable faster insights and decision-making in complex global trials.
Asia Pacific region is poised to be the fastest growing market for electronic trial master file (eTMF). Several developing nations like China, India, and South Korea are increasingly becoming global clinical trial destinations, owing to lower costs and a large patient pool.
For instance, according to a report by the Clinical Leader, clinical trials in Australia are also 30%-40% less expensive than in the U.S. and Europe, and this cost differential spans the entire research process, from centralized and local laboratories to doctor and hospital costs. Australia also offers a diverse patient population and a top-notch medical infrastructure system.
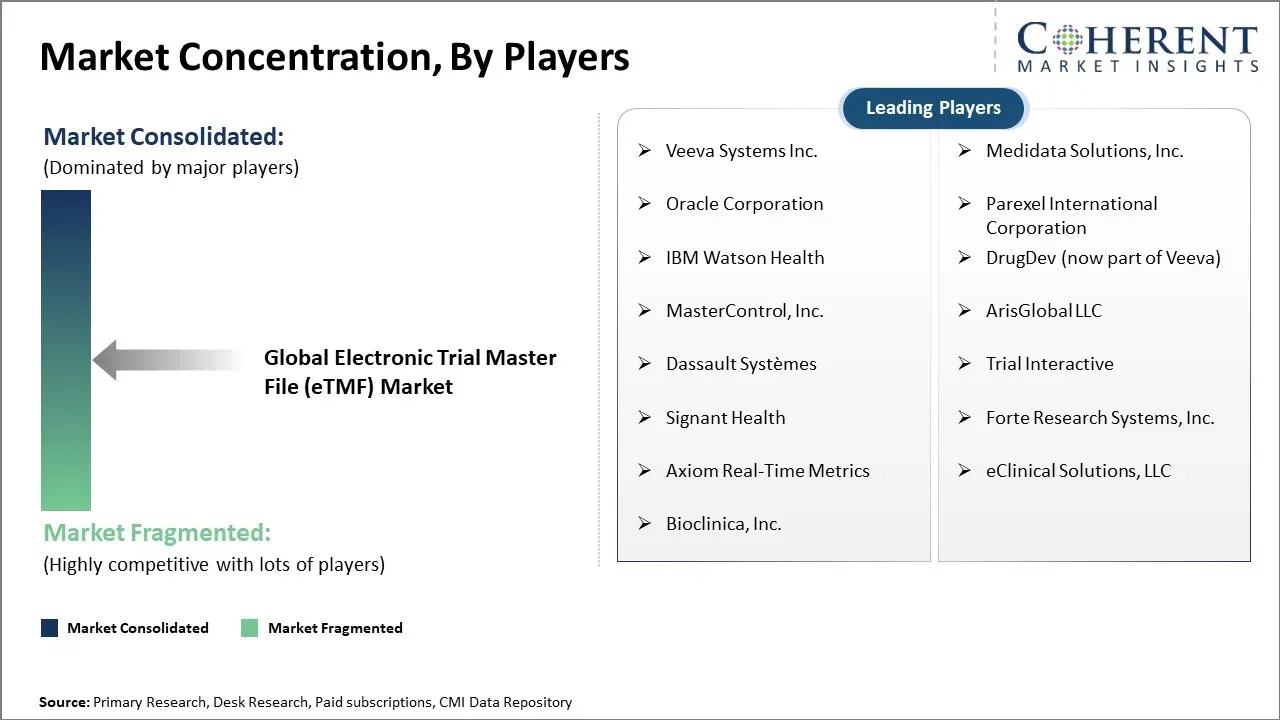
In the U.S., leading eTMF providers like Veeva Systems, Phlexglobal, and IQVIA hold significant market share, serving major pharmaceutical companies and contract research organizations (CROs).
These companies continuously invest in R&D to comply with stringent U.S. Food and Drug Administration regulations such as 21 CFR Part 11, focusing on features like automated audit trails, real-time inspection readiness, and enhanced security protocols. Their platforms are widely adopted due to proven scalability, regulatory compliance, and integration with broader clinical trial management systems.
China’s rapidly expanding pharmaceutical R&D sector and increasing number of clinical trials conducted domestically are major drivers for eTMF adoption. The National Medical Products Administration (NMPA) has modernized regulations to align more closely with international standards, pushing Chinese sponsors and CROs to adopt electronic document management systems to enhance compliance and efficiency. Furthermore, government initiatives supporting innovation and digital health infrastructure contribute to increased investment in eTMF technologies, positioning China as a high-growth market.
Japan’s pharmaceutical industry prioritizes high-quality clinical data management and rapid time-to-market for new therapies, driving demand for sophisticated eTMF solutions. The Japanese regulatory authority, Pharmaceuticals and Medical Devices Agency (PMDA), mandates rigorous documentation standards, prompting local companies to adopt electronic systems that streamline trial master file management and inspection readiness. Additionally, Japan’s increasing participation in global multi-regional clinical trials fuels the need for interoperable and compliant eTMF platforms.
Canada’s growing clinical research sector is increasingly adopting eTMF systems driven by Health Canada’s stringent requirements for data integrity and audit readiness. With a strong focus on patient safety and compliance, Canadian sponsors and CROs prioritize eTMF solutions that ensure secure, tamper-proof documentation and facilitate regulatory inspections.
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD USD 2.09 Bn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 12.6% | 2032 Value Projection: | USD USD 4.81 Bn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Veeva Systems Inc., Medidata Solutions, Inc., Oracle Corporation, Parexel International Corporation, IBM Watson Health, DrugDev (now part of Veeva), MasterControl, Inc., ArisGlobal LLC, Dassault Systèmes, Trial Interactive, Signant Health, Forte Research Systems, Inc., Axiom Real-Time Metrics, eClinical Solutions, LLC, and Bioclinica, Inc. |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Proprietary Elements
*Definition: Global electronic trial master file (eTMF) market provides a centralized, cloud-based platform for organizations to electronically organize, store, manage, track, and access all essential documentation for clinical trials in a digital format. eTMF systems help optimize clinical trials by facilitating compliance with regulatory guidelines, improving collaboration between research sites and sponsors, and enhancing the overall efficiency of clinical development programs.
Share
Share
About Author
Monica Shevgan has 9+ years of experience in market research and business consulting driving client-centric product delivery of the Information and Communication Technology (ICT) team, enhancing client experiences, and shaping business strategy for optimal outcomes. Passionate about client success.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients