e-Clinical Solutions – Leveraging Clinical Trial Data to Decrease Risk, Time, and Cost
eClinical solutions is used to improve the clinical development process through data management and data analysis. It offers total transparency and utilization of the clinical and operational data by providing data management software and customized data management services, including electronic data capture (EDC), clinical reporting, clinical data repository platform and data management and standardization. The advantages and applications of eClinical software allows users to analyze, manage, integrate, and standardize all their clinical and operational data with the help of integrated advanced visualization and analytical capabilities. Software such as clinical trial managements system (CTMS) help users to comply with government regulations, patient management, investigator management, budgeting, and adverse event reporting system among others.
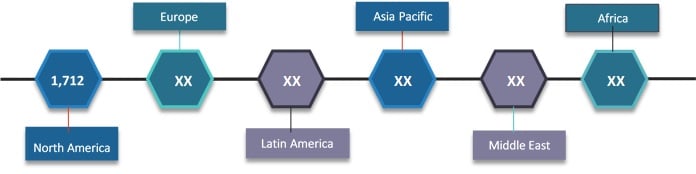
The global eClinical solutions market was valued at US$ 3.5 billion in 2017 and is expected to witness a robust CAGR of 11.2% over the forecast period (2018 – 2026).
Figure 1. Global eClinical Solutions Market Value (US$ Mn), by Region, 2018
To learn more about this report, Download Free Sample
Rapid Research and Development in eClinical field by biotechnology and pharmaceutical companies is expected to drive growth of the eClinical solutions market
Various software application of clinical solutions are gaining acceptability in clinical trials, which is supposed to be the key factor driving growth of the eClinical solutions market. The other factors such as increasing government funding and rising R&D programs by biopharma and pharmaceutical companies are expected to produce new products, which will further boost growth in eClinical solutions market. Innovations and technological advances in the field of software solutions will help in fuelling growth in the market over the forecast period. According to U.S. National Library of Medicine, in last 20 years, there has been a significant increase in the number of new clinical trials registered every year.
Extensive research and development has led the companies to outsource R&D activities from other cheaper avenues. The independent service providers such as CROs are committed to provide high quality and timely data, adhering to the regulatory guidleines and post-approval regulatory requirements, which in turn facilitates in lowering the overall cost. For instance, in 2011, Pfizer entered into an agreement with Parexel and Icon. Under this agreement, Parexel provided Pfizer with a range of clinical development services for five years.
Rising private and public funding to support clinical trials
Pharmaceutical companies and government funding agencies are increasingly funding various clinical trials. According to the Journal of the American Medical Association 2015, the number of clinical trials sponsored by companies were 6,550 and 1,048 by the NIH. In the U.K., the government funds health related research through organizations such as the National Institute for Health Research (NIHR) and the Medical Research Council (MRC). They also help to coordinate cancer research nationally through the National Cancer Research Institute (NCRI). NCI funds around half of all cancer trials in the U.S. According to Research America, in the U.S., investment in health and medical R&D grew by 13.3% from 2013 to 2015. However, high implementation cost of software, lack of skilled professional, data privacy issues, and low awareness about the software are key factors hampering eClinical solutions market growth.
Key players operating in the eClinical solutions market include BioClinica Inc., CRF Health, DATATRAK International Inc., eClinical Solutions Inc., MaxisIT Inc., Medidata Solutions Inc., Merge Healthcare Inc., OmniComm Systems Inc., Oracle Co., and PAREXEL International Co.
Share
Share
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients