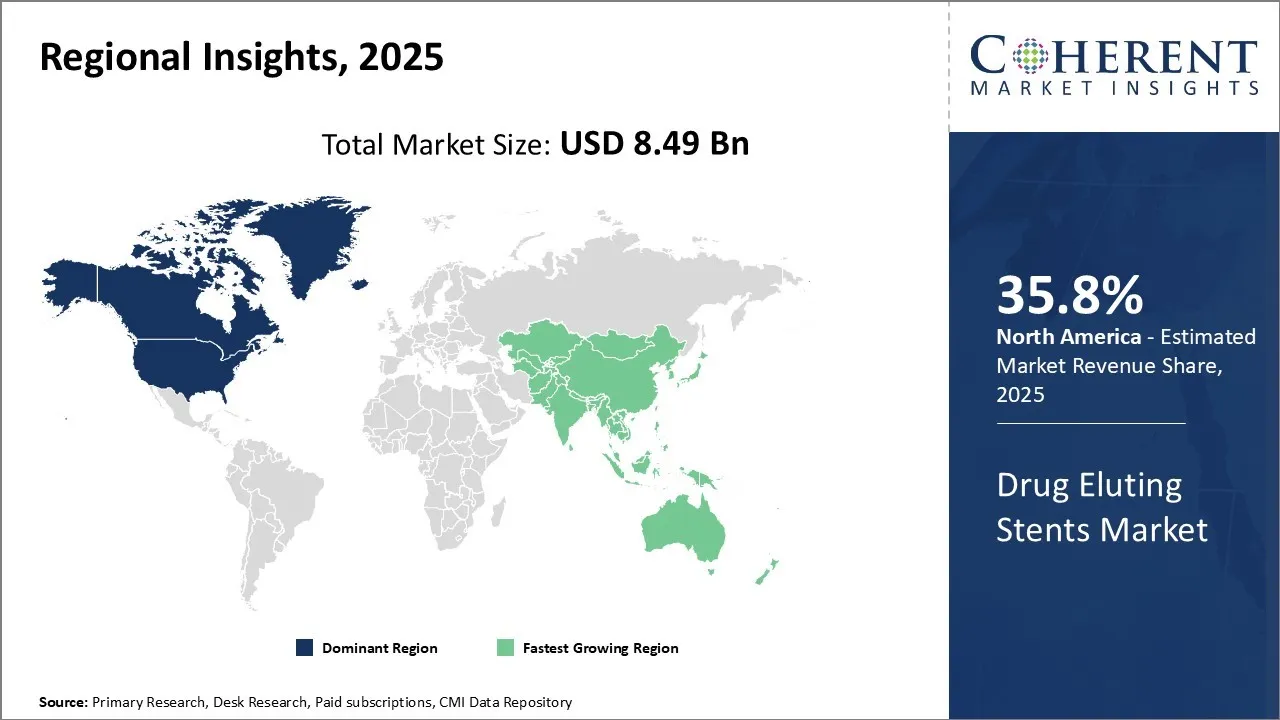
The Global Drug Eluting Stents Market share is estimated to be valued at USD 8.49 Bn in 2025 and is expected to reach USD 15.22 Bn by 2032 exhibiting a compound annual growth rate (CAGR) of8.7% from 2025 to 2032.
The global drug eluting stents market demand is expected to witness a positive growth trend over the forecast period. Factors such as rising prevalence of cardiovascular diseases, growing geriatric population, rising healthcare expenditures, increasing adoption of minimally invasive surgeries and interventional cardiology are expected to drive the drug eluting stents market growth during this period. Moreover, ongoing technological advancements in the field of drug eluting stents including bioresorbable stents and nano-technology coatings are further expected to provide new growth opportunities to market players in the coming years. However, factors such as stringent regulatory approval process and product recalls may hinder the market growth to some extent during the forecast period.
|
Current Event |
Description and its Impact |
|
Rising Global Cardiovascular Disease Burden |
|
|
Technological Advancements |
|
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Reimbursement plays a critical role in the adoption and accessibility of drug-eluting stents (DES) across global healthcare systems. Approval for reimbursement is often contingent on demonstrated clinical efficacy, cost-effectiveness, and real-world outcomes. A key example is Abbott's XIENCE V Everolimus Eluting Coronary Stent System, which received reimbursement approval in France after becoming the first DES to show clinical superiority over another DES in a randomized clinical trial. This milestone highlights how strong clinical evidence can support favorable reimbursement decisions, driving wider adoption and market penetration for advanced DES technologies.
In terms of product type, the polymer-based coatings segment is expected to contribute the highest share of the market with 52.6% in 2025 owning to continuous technological advancements that have enhanced the material properties of polymers used for coating drug-eluting stents. The introduction of new generation polymers that are biocompatible and allow for controlled and sustained drug release has increased the appeal of polymer-based coatings. The polymers now mimic characteristics of natural tissues and offer reduced inflammatory response post implantation. This has translated to better clinical outcomes for patients and higher acceptance amongst clinicians. Ongoing R&D is further exploring the potential of smart polymers whose drug release properties can be stimulated in response to specific bio signals. These smart coatings are expected to tremendously benefit personalized therapy and treatment compliance.
In November 2024, a bioadaptive coronary stent introduced at TCT 2024 has the potential to significantly diminish long-term target lesion failure, a challenge associated with earlier-generation implants. Unlike traditional drug-eluting stents, this innovative design is capable of dynamically adjusting to the artery over time, which may help to prevent the gradual deterioration of vessel patency.
In terms of application, the coronary artery disease segment is expected to contribute the highest share of the market with 43.7% in 2025 owing to rising prevalence globally. Coronary artery disease is one of the leading causes of mortality and a major economic burden. With growth in geriatric population and lifestyle changes, the risks of atherosclerosis and plaque formation in arteries have surged tremendously. In developing nations, adoption of sedentary urban lifestyles coupled with growing consumption of junk and processed foods has substantially increased metabolic disorders as well as lifestyle diseases. This has contributed to surging incidences of coronary artery blockages and subsequent procedures like angioplasty with stenting. The clinical benefits of drug-eluting stents over bare-metal stents in reducing restenosis rate post coronary intervention has augmented their demand among CAD patients.
In December 2023, Terumo India, a subsidiary of the Japan-based Terumo Corporation, launched the Ultimaster Nagomi™ drug-eluting stent (DES) for the treatment of coronary artery disease (CAD). This innovative device is designed to facilitate seamless navigation within both small and large coronary vessels, providing customized stenting solutions that cater to a wider patient demographic.
In terms of end user, the hospitals segment is expected to contribute the highest share of the market with 40.8% in 2025, driven by large patient volumes treated at hospitals along with reimbursement benefits. As a majority of stenting procedures are performed at hospital cath-labs by trained intervention cardiologists, hospitals account for the bulk of drug-eluting stent sales. In addition, most universal healthcare systems follow favorable reimbursement practices for in-hospital treatments compared to other healthcare facilities. This makes stenting procedures more affordable for patients at hospitals. Furthermore, hospitals have better infrastructure and trained staff to handle post-procedural care as needed. This helps lower complications risks compared to other end users, boosting overall preference and demand for drug eluting stents at hospitals.
To learn more about this report, Download Free Sample
North America’s dominance in the drug eluting stents market with share of 35.8% in 2025 can be attributed to factors such as the well-established healthcare infrastructure, growing prevalence of cardiovascular diseases, and presence of leading market players. According to Heart Disease and Stroke Prevention, Coronary heart disease (CHD) is the most common type of heart disease, killing approximately 375,476 people annually. Every year about 805,000 Americans have a heart attack. Drug-eluting stents (DES) are needed by heart disease patients to prevent restenosis, which is the re-narrowing of a coronary artery after it has been widened with a stent. This is further accelerating the drug eluting stents market demand.
The Asia Pacific region exhibits the fastest growth with share of 28.2% in 2025 and is expected to witness significant expansion over the forecast period. This can be credited to the improving access to healthcare in developing nations, rising medical tourism, and increasing healthcare expenditures. Additionally, there is a significant rise in the cardiovascular disease burden observed in Asia Pacific region and supportive government initiatives. Heart disease is the leading cause of death in countries like India, China, Japan, and South Korea. For instance, China alone accounts for over 40% of global PCI volume, driving major DES usage. Regulatory bodies in India (CDSCO), China (NMPA), and Japan (PMDA) are fast-tracking approvals for newer-generation DES.
The U.S. dominated the drug eluting stents market with, driven by strong healthcare infrastructure, high adoption of advanced technologies. U.S. is leading the DES innovation and implementation. For instance, in March 2024, Boston Scientific gained FDA approval for its Agent paclitaxel-coated balloon catheter, the first device indicated to treat coronary in‑stent restenosis (ISR) without placing another stent. This is further proliferating the drug eluting stents market share.
Japan’s aging population significantly drives the demand for drug eluting stents. In February 2023, the Japanese Ministry of Health reported an increase in funding for cardiovascular treatments, including drug eluting stents, to address the growing health needs of the elderly. Additionally, the local manufacturers of Japan are now ramping up R&D, focusing on ultrathin-strut, polymer-free, and bioresorbable DES designs that align with Japan’s strict quality standards. A 2023 survey showed 86.5% of Japanese interventional cardiologists routinely use Xience everolimus-eluting stents, indicating strong clinical trust in established DES brands. This is further accelerating the drug eluting stents market growth.
Germany drug eluting stents market benefits from strong government support for cardiovascular health initiatives. In March 2023, the German Federal Ministry of Health announced funding for innovative medical technologies, including drug eluting stents, to improve patient care and outcomes. Moreover, Germany is a leading adopter and innovator in DES technology. High clinical usage, superior patient outcomes, and active R&D into biodegradable stents underscore the country’s dynamic role in advancing interventional cardiology.
India's market for drug eluting stents is expanding due to rising healthcare investments and increasing awareness about cardiovascular diseases. In April 2023, the Indian Council of Medical Research launched a national initiative to promote the use of drug eluting stents for better management of heart diseases. Furthermore, India is expanding its Drug eluting stent andscapre through rapid adoption, supportive ecosystem and great innovation. For instance, Indian manufacturers such as Sahajanand Medical Technologies (Supraflex Cruz) and Translumina (VIVO ISAR DDCS) are producing advanced, polymer-free, dual-drug DES, now approved in global markets including Australia. Insurance schemes such as Ayushman Bharat and expansion of cath labs in tier-2/3 cities are increasing patient access. This is further proliferating drug eluting stents market revenue.
To learn more about this report, Download Free Sample
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 8.49 Bn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 8.7% | 2032 Value Projection: | USD 15.22 Bn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
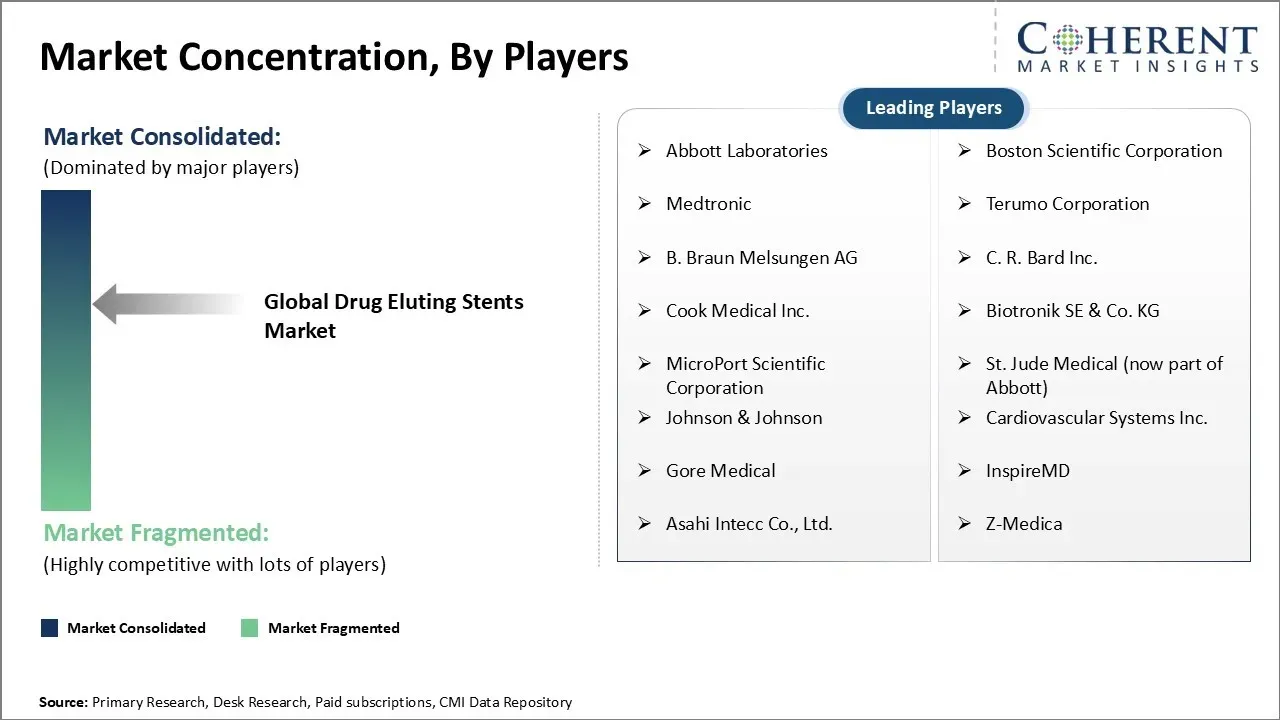
Abbott Laboratories, Boston Scientific Corporation, Medtronic, Terumo Corporation, B. Braun Melsungen AG, C. R. Bard Inc., Cook Medical Inc., Biotronik SE & Co. KG, MicroPort Scientific Corporation, St. Jude Medical (now part of Abbott), Johnson & Johnson, Cardiovascular Systems Inc., Gore Medical, InspireMD, Asahi Intecc Co., Ltd., and Z-Medica |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
The rising incidence of cardiovascular diseases across major parts of the world has been a key factor fueling demand for drug eluting stents in recent years. Heart diseases have emerged as one of the biggest causes of mortality, with an increasing number of people suffering from conditions like coronary artery disease, coronary heart disease, and strokes. Some of the important factors responsible for the growing cases of CVDs include sedentary lifestyles, unhealthy diets, obesity, high cholesterol, diabetes, and high blood pressure which have become highly prevalent.
According to the National Institute of Health, Cardiovascular disease (CVD) remains the leading cause of death globally, with approximately 20 million fatalities each year, accounting for nearly one in five deaths worldwide. Recent drug eluting stents market research highlights this surge in demand, with innovations like Elixir Medical’s DynamX Bioadaptor unveiled at EuroPCR 2025 demonstrating significantly lower target lesion failure and cardiovascular death rates compared to traditional drug-eluting stents, reflecting the growing shift toward next-generation interventional cardiology solutions.
The global drug eluting stents market is witnessing opportunities due to the growing demand for minimally invasive procedures. Minimally invasive procedures such as coronary angioplasty with drug eluting stent implantation allow faster recovery and cause minimal pain and trauma to patients as compared to conventional open-heart surgeries. With rising health awareness and easier access to information, more and more patients are opting for minimally invasive treatments. This trend is working in favor of the drug eluting stents market. Additionally, the aging population that is more prone to cardiac issues also prefers minimally invasive treatments. According to recent drug eluting stents market forecast reports, these factors are expected to significantly drive market growth over the next several years.
The Drug-Eluting Stents (DES) market value is undergoing a pivotal transformation, driven by continuous innovation, intensifying competition, and proactive clinical revalidation strategies from established industry players. The current competitive landscape emphasizes not only technological advancements but also long-term clinical outcomes, cost-effectiveness within value-based care frameworks, and the exploration of complex anatomies that were previously deemed inoperable for DES.
The era of incremental stents improvements has concluded. Companies that relying on marginal enhancements or minor modifications to polymer are quickly becoming overshadowed. The market is bifurcating between players who are advancing next-generation bioresorbable polymer platforms with tailored elution kinetics and those still recycling first-gen cobalt-chromium formulations with limited differentiation.
For instance, Abbott’s Xience Sierra and Medtronic’s Resolute Onyx maintain their strong clinical position due to their robust multi-year follow-up data and lower incidence of stent thrombosis, particularly among diabetic patients, a demographic with a significantly high-risk of restenosis. A 5-year analysis from the EVOLVE II trial showed that Boston Scientific's Synergy stent achieved target lesion failure rates of only 8.3%, significantly outperforming legacy bare-metal and first-gen DES platforms.
However, the narrative of true disruption is centered on bioresorbable technologies. While initial enthusiasm diminished after the first failures like the Absorb BVS, the current generation, represented by Magmaris (BIOTRONIK) and MeRes100 (Meril Life), is showing renewed potential. Clinical data from the MeRes-1 trial (3-year follow-up) demonstrated late lumen gain without the chronic inflammation associated with permanent polymer-based stents, a proposition that is both clinically and economically appealing for long-term care providers.
From a regulatory perspective, the FDA’s increasing demand for longer-term (5+ year) safety data before granting approvals, which is altering product launch timelines. Companies that possess substantial real-world evidence and robust post-market surveillance infrastructure are likely to prevail, not those relaying olely on impressive trial results from narrowly defined patient populations. Regulatory bodies in countries such as Japan and Germany are already mandating cost-effectiveness assessments as a prerequisite for reimbursement, favoring stents that feature dual-layer designs and demonstrate proven outcomes in multivessel disease.
Share
Share
About Author
Komal Dighe is a Management Consultant with over 8 years of experience in market research and consulting. She excels in managing and delivering high-quality insights and solutions in Health-tech Consulting reports. Her expertise encompasses conducting both primary and secondary research, effectively addressing client requirements, and excelling in market estimation and forecast. Her comprehensive approach ensures that clients receive thorough and accurate analyses, enabling them to make informed decisions and capitalize on market opportunities.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients