Global Drug Device Combination Product Market is estimated to be valued at US$ 150.23 billion in 2023 and is expected to exhibit a CAGR of 7.6% during the forecast period (2023-2030).
Analysts’ Views on Global Drug Device Combination Product Market:
The global drug device combination product market is characterized by a diverse range of products, including drug-eluting stents, infusion pumps, inhalers, and insulin pens, among others. These innovative products offer improved patient outcomes, enhanced convenience, and targeted drug delivery. The market is witnessing significant investments in research and development, aiming to introduce novel combinations and expand the therapeutic applications. Furthermore, the market is driven by the rising demand for personalized medicine and the increasing adoption of combination products in the treatment of chronic diseases, such as cardiovascular disorders, diabetes, and respiratory conditions. As the market continues to evolve, collaborations between pharmaceutical and medical device companies, along with regulatory advancements, are expected to drive further growth and innovation.
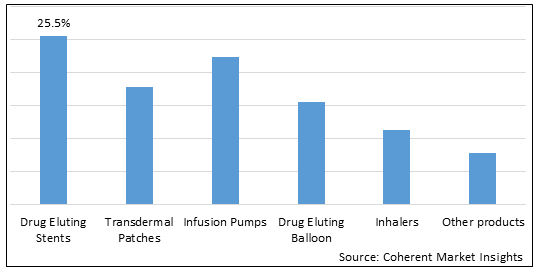
Figure 1. Global Drug Device Combination Product Market Share (%), By Products Type, 2023
To learn more about this report, Download Free Sample
Global Drug Device Combination Product Market– Driver
Increased product launches
The rising burden of chronic disorders, such as cardiovascular diseases, cancers, chronic respiratory diseases, and diabetes, is fueling the growth of the drug device combination product market. For instance, on January 4th 2023, Valencell is a leading innovator in wearable biometric sensor technology, specializing in accurate and reliable physiological monitoring for a range of applications. Valencell has announced plans to launch its own branded product line in the digital health sector as it concentrates efforts to bring solutions to market to manage chronic diseases.
Government Initiatives Driving Market Growth
Governments around the world are implementing various initiatives to address the rising burden of chronic disorders and promote patient safety. These initiatives are playing a crucial role in driving the growth of the global drug device combination product market. For instance, In September 2021, the US FDA recognized the significant impact of AI/ML in drug development. Over 100 drug and biologic submissions with AI/ML components were reported this year. AI/ML is employed in various stages of drug development, including drug discovery, clinical research, safety surveillance, and manufacturing. The FDA acknowledges the potential of AI/ML in Digital Health Technologies and Real-World Data analytics
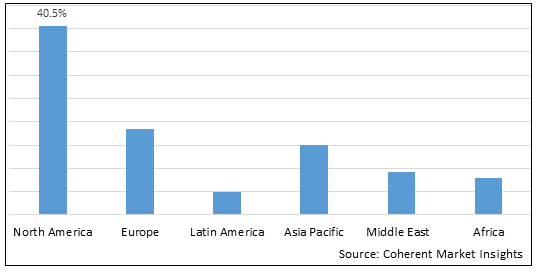
Figure 2. Global Drug Device Combination Product Market Value (US$ Million), By Region, 2023
To learn more about this report, Download Free Sample
Global Drug Device Combination Product Market - Regional Analysis
Among region, North America is estimated to hold a dominant position in the global drug device combination product market over the forecast period. North America is estimated to hold 40.51% of the market share in 2030.
Global Drug Device Combination Product Market– Impact of Coronavirus (COVID-19) Pandemic
Since the COVID-19 virus outbreak in December 2019, the disease has spread to over 100 countries across the globe, and the World Health Organization declared it a public health emergency on January 30, 2020.
COVID-19 affected the economy in three main ways: by directly affecting the production and demand of drugs, by creating disruptions in distribution channels, and through its financial impact on firms and financial markets. Due to nationwide lockdowns, several countries, such as China, India, Saudi Arabia, the U.A.E., Egypt, and others faced problems regarding transportation of drugs from one place to another.
However, the COVID-19 pandemic has had a significant impact on the global drug device combination product market. Surging Demand for Respiratory Devices and Advancements in drug-device combinations shape the Market. However, disruptions in manufacturing and supply chains, as well as resource prioritization, have posed challenges. The pandemic has driven innovation and collaboration in the industry, but the long-term impact is still unfolding.
Drug Device Combination Product Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2022 | Market Size in 20: | US$ 150.23 Bn |
| Historical Data for: | 2017 to 2021 | Forecast Period: | 2023 to 2030 |
| Forecast Period 2023 to 2030 CAGR: | 7.6% | 2030 Value Projection: | US$ 251.68 Bn |
| Geographies covered: | North America: U.S. and Canada Latin America: Brazil, Argentina, Mexico, and Rest of Latin America Europe: Germany, U.K., Spain, France, Italy, Russia, and Rest of Europe Asia Pacific: China, India, Japan, Australia, South Korea, ASEAN, and Rest of Asia Pacific Middle East & Africa: GCC, Israel, and Rest of Middle East, South Africa, North Africa, and Central Africa |
||
| Segments covered: |
|
||
| Companies covered: |
AbbVie Inc. (Allergan), Terumo Corporation, GlaxoSmithKline PLC, Novartis AG, Medtronic PLC, Abbott Laboratories, Boston Scientific Corp., W L Gore and Associates Inc., Stryker Corporation, and Becton, Dickinson and Company, |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Global Drug Device Combination Product Market - Segmentation
The global drug device combination product market report is segmented into product type, Application, End user, and Region.
By Products Type, the market is segmented into Drug Eluting Stents, Transdermal Patches, Infusion Pumps, Drug Eluting Balloon, Inhalers, and Other Products. Drug eluting stents (DES) have emerged as the dominant segment in the global drug device combination product market, primarily due to their superior outcomes, technological advancements, and robust clinical evidence. DES is the preferred choice for treating cardiovascular diseases, supported by high demand, continuous innovations, and regulatory acceptance. These factors have solidified the position of DES as the leading product type in the market. For instance, on May 13, 2022, Medtronic plc, announced U.S. FDA approval for the Onyx Frontier drug-eluting stent. This latest advancement in stent technology enhances deliverability and acute performance in treating coronary artery disease (CAD), showcasing Medtronic's commitment to innovative solutions in interventional cardiology.
By Application, the market is segmented into Cardiovascular, Diabetes, Cancer Treatment, Respiratory Diseases, and Other Applications. The global drug device combination product market is primarily driven by the cardiovascular treatment segment, which holds a dominant position. This is attributed to the widespread occurrence of cardiovascular diseases, the significant demand for efficient treatment options, continuous progress in drug-device combinations, robust clinical evidence supporting their effectiveness, and favorable regulatory and reimbursement frameworks.
By End User, the market is segmented into Hospitals, Ambulatory Surgical Centers, and Other End Users. Hospitals play a pivotal role in the utilization of drug device combination products, primarily due to the larger patient population and capacity to handle intricate medical procedures. Hospitals are prominent position in the market is reinforced by their ability to provide comprehensive healthcare services within a single facility, catering to diverse medical needs.
By Region, the market is segmented into North America, Europe, Latin America, Asia-Pacific, Middle East and Africa, and Rest of the world. The global drug device combination product market has traditionally witnessed dominance in North America and Europe. These regions boast well-established healthcare systems, advanced medical technology infrastructure, and robust regulatory frameworks that foster the development and commercialization of drug device combination products.
Global Drug Device Combination Product Market - Cross Sectional Analysis
Among products type, Drug eluting stents (DES) held a dominant position in North America region over the forecast period due to increasing number of inorganic growth strategies such as collaboration and others by the market players for product development. For instance, 13 May, 2022, Medtronic plc, a global leader in healthcare technology, announced it received U.S. Food and Drug Administration (FDA) approval for the Onyx Frontier drug-eluting stent (DES).
Global Drug Device Combination Product Market: Key Developments
On February 19, 2020, Flowonix Medical, Inc., received U.S. FDA approval to market the Prometra II Pump System for intrathecal baclofen delivery. This device offers expanded treatment options, with 20 mL and 40 mL capacities, and features a valve-gated mechanism for comprehensive spinal cord coverage. With a battery life of over 10 years, it reduces the need for pump replacements. This development provides new possibilities for managing severe spasticity in conditions, such as cerebral palsy and multiple sclerosis. Flowonix aims to establish the Prometra System as the gold standard in intrathecal baclofen therapy through collaborations with leading physicians.
On September 14, 2022, Terumo Cardiovascular Terumo manufactures the longest running line of commercially available heart-lung machines in the world, and is a leader in quality systems management. Terumo Cardiovascular opens a new manufacturing facility in Costa Rica, investing US$42 million to produce perfusion circuits for cardiovascular surgeries. The expansion supports global customers, improves patient care, and takes advantage of Costa Rica's stable economy and skilled workforce. The facility commenced operations in August 2022 and aims to employ around 300 associates by year-end.
On November 14, 2022, Stryker is one of the world’s leading medical technology company. Stryker introduced the OR (operating room model) of the future, a model operating room in Flower Mound, Texas. It offers customers an interactive experience to explore advanced OR (operating room model) design and technology. The model prioritizes patient and staff safety, productivity, and operational efficiency, incorporating features for infection prevention, clean ability, and intelligent technology. It showcases seamlessly integrated software and equipment, sterility enhancements, and personalized patient care through continuous data generation. The design elements aim to save time and cost during construction and allow for future upgrades. Stryker aims to empower surgical teams and deliver high-performing ORs (operating room model) that enhance patient care.
On May 22, 2023, Becton, Dickinson and Company (BD), BD is one of the largest global medical technology companies in the world and is advancing the world of healthTM by improving medical discovery. BDannounced the worldwide commercial launch of the BD FACSDiscover S8 Cell Sorter, a new-to-world cell sorting instrument. It incorporates two breakthrough technologies, BD SpectralFX Technology and BD CellView Image Technology, which enable researchers to obtain more detailed information about cells that were previously invisible in traditional flow cytometry experiments.
Global Drug Device Combination Product Market - Key Trends
Drug-Eluting Stents to Drive Significant Growth in drug device combination products market
The global drug device combination products market is witnessing notable growth, driven by the increasing adoption of drug-eluting stents (DES). These stents, made of metal and coated with medication, effectively block cell proliferation, setting a new standard of care. With different generations of DES available, the market is witnessing increased demand due to the rising prevalence of cardiovascular diseases. Furthermore, frequent product developments and approvals by key market players are contributing to the market's growth trajectory. For instance, in August 2021, SINOMED and NUI Galway, SINOMED is a global company engaged in patient-focused medical innovations for interventional medicine. SINOMED and NUI Galway collaborated to successfully implant the HT Supreme Drug-Eluting Stent (DES) at University Hospital Galway. This innovative stent enables interventional cardiologists to treat patients with coronary artery disease and small vessels that were difficult to treat before.
Global Drug Device Combination Product Market: Restraint
Product Recalls and Stringent Regulations Pose Challenges to Global Drug Device Combination Product Market
The growth of the global drug device combination product market faces obstacles as product recalls and associated complications arise. Instances of adverse events and harm have led to recalls of certain drug device combination products, impacting market growth. Additionally, stringent government regulations and policies aimed at ensuring safety and efficacy have been introduced, creating hurdles for the development and marketing of these products. These challenges emphasize the importance of quality control and regulatory compliance in the industry.
Risk of Infections
The risk of infections associated with drug-device combination products poses a significant constraint on market growth. For instance, a 66-year-old woman who underwent knee replacement surgery developed a recurring infection, resulting in multiple surgeries and ultimately the amputation of her leg. This case underscores the widespread problem of infections related to implanted medical devices. To address this issue, researchers from Binghamton University, Stevens Institute of Technology, Syracuse University, and City College of New York are collaborating to develop infection-resistant tissue scaffolds that can prevent biofilm formation and eliminate medical device-associated infections.
Strict Regulations
Strict and non-specific regulatory pathways for drug-device combination product approval further hinder market growth. In the United States, pharmaceutical products and medical devices follow separate regulatory approval processes. For instance, in September 4, 2020, The U.S. FDA's Center for Devices and Radiological Health (CDRH) regulates medical devices and radiation-emitting electronic products in the United States. It oversees requirements for establishment registration, device listing, premarket notification (510(k)), quality system regulation, labeling, and medical device reporting. The CDRH ensures the safety and effectiveness of these products.
Global Drug Device Combination Product Market- Key Players
Major players operating in the global drug device combination product market include AbbVie Inc. (Allergan), Terumo Corporation, GlaxoSmithKline PLC, Novartis AG, Medtronic PLC, Abbott Laboratories, Boston Scientific Corp., W L Gore and Associates Inc., Stryker Corporation, and Becton, Dickinson and Company,
Definition: The Global Drug Device Combination Product Market refers to the market segment that encompasses medical products combining drugs and devices in a single entity. These combination products aim to provide enhanced therapeutic benefits, improved patient outcomes, and increased convenience by integrating the functionalities of drugs and medical devices. They can include various forms such as drug-eluting stents, inhalers, insulin delivery systems, and infusion pumps, among others. The market is driven by advancements in technology, increasing prevalence of chronic diseases, and the growing need for targeted and personalized treatments. Regulatory frameworks and collaborations between pharmaceutical and medical device companies play a crucial role in shaping the development, commercialization, and adoption of these innovative products in healthcare settings worldwide.
Share
Share
About Author
Manisha Vibhute is a consultant with over 5 years of experience in market research and consulting. With a strong understanding of market dynamics, Manisha assists clients in developing effective market access strategies. She helps medical device companies navigate pricing, reimbursement, and regulatory pathways to ensure successful product launches.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients