Dravet Syndrome Treatment Market Size and Forecast – 2025 – 2032
The Global Dravet Syndrome Treatment Market size is estimated to be valued at USD 1.2 billion in 2025 and is expected to reach USD 2.8 billion by 2032, exhibiting a compound annual growth rate (CAGR) of 13.5% from 2025 to 2032.
Global Dravet Syndrome Treatment Market Overview
Dravet syndrome treatments focus on managing severe, drug-resistant epileptic seizures typically beginning in infancy. The product portfolio includes antiepileptic drugs (AEDs), cannabinoid-based therapies, and novel gene-targeted treatments. FDA-approved options such as stiripentol, fenfluramine, and cannabidiol have significantly improved seizure control and patient outcomes.
Adjunct therapies, including vagus nerve stimulation devices and ketogenic diets, are also used to enhance seizure management. Recent innovations involve gene therapy and precision medicine approaches aiming to address the underlying genetic mutations, primarily in the SCN1A gene, offering new hope for long-term disease modification.
Key Takeaways
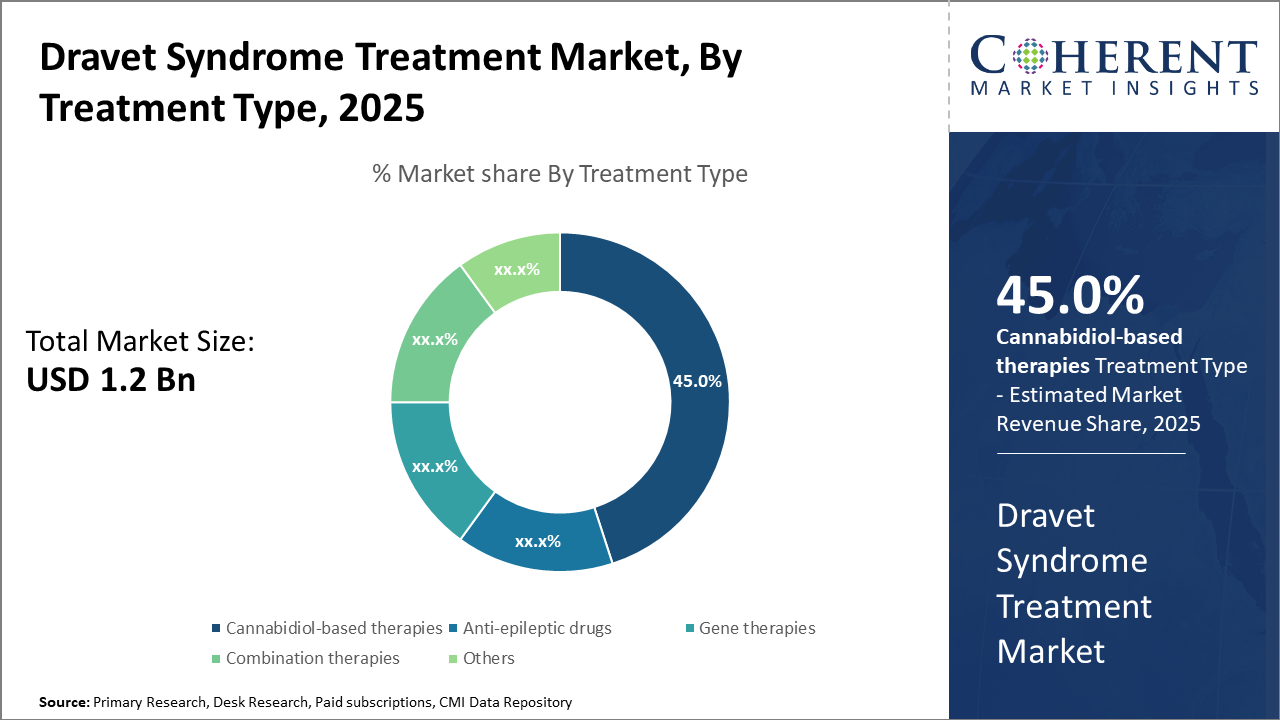
The cannabidiol-based therapy segment holds the largest market share in treatment type, driven by regulatory approvals and patient preference for non-traditional options.
Hospital pharmacies dominate the distribution channel segment due to preferred accessibility and professional oversight.
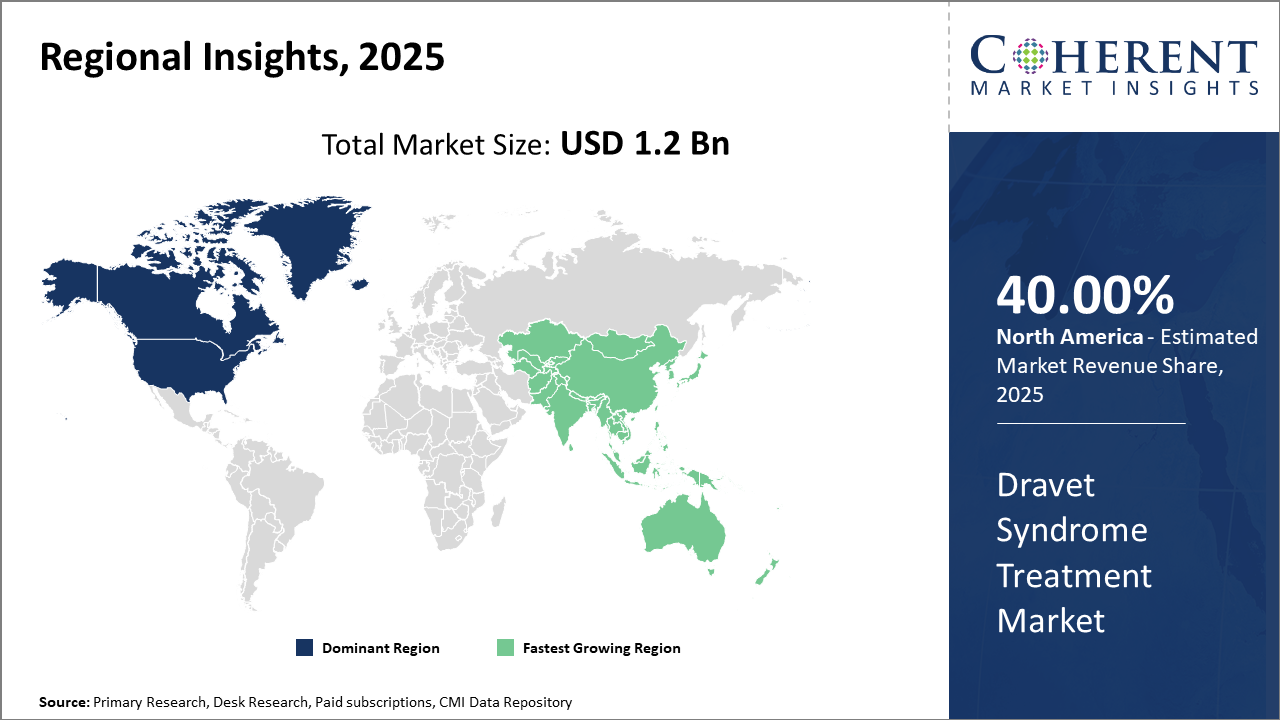
North America leads in market revenue with a robust infrastructure supporting innovative therapeutic launches and higher adoption rates.
Asia Pacific shows the fastest CAGR, primarily fueled by improved healthcare infrastructure and government initiatives promoting rare disease diagnosis
Dravet Syndrome Treatment Market Segmentation Analysis
To learn more about this report, Download Free Sample
Dravet Syndrome Treatment Market Insights, By Treatment Type
Cannabidiol-based therapies dominate the market share with approximately 45%, propelled by well-documented efficacy and regulatory endorsements. This segment benefits from widespread physician adoption and increased patient acceptance, particularly for pediatric cases. Gene therapies represent the fastest-growing subsegment, fueled by breakthroughs in genetic correction methods promising durable seizure control. Anti-epileptic drugs remain a significant segment owing to established clinical use and broad availability, serving as a foundational treatment modality.
Dravet Syndrome Treatment Market Insights, By Application
In terms of application, Pediatric use accounts for the largest market share, reflecting the early onset characteristic of Dravet syndrome and the priority of early intervention strategies. The demand in this subsegment is predominantly driven by caregivers’ focus on improving quality of life and reducing seizure incidents. Adult use is the fastest-growing subsegment as improved treatment options extend life expectancy and enhance adult patient management. Geriatric use remains limited due to the rarity of syndrome manifestation at advanced ages, but it is slowly gaining attention amid longer survival rates.
Dravet Syndrome Treatment Market Insights, By Distribution Channel
Hospital pharmacies hold the dominant market share of about 55%, attributable to the necessity of professional oversight for specialized treatment administration and monitoring. These settings also facilitate access to newly launched therapies through hospital formularies and clinical trial networks. Online pharmacies represent the fastest-growing channel, driven by increased digital health adoption and improved accessibility, especially in underserved regions. Retail pharmacies maintain steady demand, primarily serving maintenance therapies and refill prescriptions.
Dravet Syndrome Treatment Market Trends
The ongoing evolution towards gene therapies has transformed the Dravet Syndrome Treatment Market by introducing more effective long-term solutions.
An example includes the progress of Stoke Therapeutics’ ASO-based treatments, which showed a 45% seizure reduction in recent Phase 2 trials.
Additionally, the surge in CBD-based product approvals globally, especially post-2024 FDA expansions, has positioned cannabidiol as a mainstream therapeutic.
The integration of digital health platforms for seizure monitoring and therapy adherence further exemplifies the confluence of technology and treatment paradigms, signifying a market shift towards holistic disease management.
Dravet Syndrome Treatment Market Insights, By Geography
To learn more about this report, Download Free Sample
North America Dravet Syndrome Treatment Market Analysis and Trends
In North America, the dominance in the Dravet Syndrome Treatment Market is bolstered by a comprehensive healthcare ecosystem, a strong regulatory framework, and the presence of key market companies such as GW Pharmaceuticals and Zogenix. The region commands nearly 40% market share in 2025, supported by favorable government policies and reimbursement schemes that facilitate patient access to innovative treatments.
Asia Pacific Dravet Syndrome Treatment Market Analysis and Trends
Meanwhile, the Asia Pacific exhibits the fastest growth with a CAGR exceeding 16%, driven by expanding healthcare infrastructure, rising awareness of rare genetic epilepsies, and increased clinical trial activity in countries like China and India. Favorable government initiatives aimed at rare disease research and emerging local pharmaceutical firms also contribute to the region’s rapid market growth.
Dravet Syndrome Treatment Market Outlook for Key Countries
USA Dravet Syndrome Treatment Market Analysis and Trends
The USA’s market is pivotal, representing the largest revenue contributor in 2025 due to an extensive patient population and advanced therapeutic approvals, including Epidiolex and promising gene therapies. The 2024 expansion of insurance coverage for rare disease treatments has significantly enhanced treatment accessibility, while ongoing FDA fast-track designations continue to accelerate pipeline products. Market players such as GW Pharmaceuticals and Zogenix actively collaborate with academic institutions to develop next-gen therapies, consolidating the U.S. market's role as an innovation hub.
Germany Dravet Syndrome Treatment Market Analysis and Trends
Germany’s market benefits from a strong healthcare infrastructure and government funding toward rare neurological disease research. Precision medicine adoption is accelerating, with local players partnering with global firms to expand compassionate use programs. The German reimbursement system’s flexibility for orphan drugs ensures substantial patient access to novel treatments, driving market revenue growth. Furthermore, the presence of renowned research centers facilitates ongoing clinical trials advancing the treatment landscape.
Analyst Opinion
The escalating demand for precision medicine treatments tailored to genetic epilepsies marks a pivotal growth driver. Recent clinical trials in 2024 showed that gene therapies targeting mutations in the SCN1A gene significantly reduced seizure frequency by over 60% in 40% of patients, underscoring the potential for personalized approaches to enhance patient outcomes. Such therapy innovations are expected to increase the market share of advanced treatments over traditional anti-epileptic drugs.
Cannabidiol (CBD)-based formulations continue to dominate treatment protocols due to strong clinical validation and regulatory approvals in multiple regions. Reports from 2025 indicate that CBD-based products account for nearly 45% of the total market revenue, mainly driven by their efficacy and reduced side effects compared to conventional therapies, highlighting a demand-side driver critical for market expansion.
Pricing dynamics remain a critical micro-indicator shaping market revenue. The introduction of generic formulations and patent expirations is likely to bring moderate price reductions, increasing accessibility without compromising industry share. For example, in late 2024, one of the leading CBD-based drugs experienced a 15% price cut post-patent expiry, contributing to an upswing in prescription volumes among pediatric populations.
Import-export patterns of specialized drugs reflect shifting global demand, with North America and Europe emerging as major importers of innovative treatments, while Asia-Pacific markets are boosting local manufacturing capabilities. In 2025, the U.S. witnessed a 20% increase in imported Dravet syndrome therapeutics, reflecting the growing patient base and treatment adoption, signaling a positive outlook on market size.
Market Scope
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2025 | Market Size in 2025: | USD 1.2 billion |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 13.5% | 2032 Value Projection: | USD 2.8 billion |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: | Zogenix, Inc., GW Pharmaceuticals (a subsidiary of Jazz Pharmaceuticals), Marinus Pharmaceuticals, Inc., Stoke Therapeutics, Biogen Inc., UCB Pharma, Lundbeck, Aquestive Therapeutics, Zynerba Pharmaceuticals, Amneal Pharmaceuticals, Innovus Pharmaceuticals, Neurelis, Inc. | ||
| Growth Drivers: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Dravet Syndrome Treatment Market Growth Factors
The rising prevalence of rare pediatric epilepsy disorders, including Dravet syndrome, continues to serve as a crucial market growth driver, with epidemiological studies in 2024 reporting an incidence of approximately 1 in 15,000 births worldwide. Innovation in drug development is another driving force, characterized by accelerated clinical development timelines for gene therapies and CBD-based products that capitalize on regulatory fast-track mechanisms. Increased awareness campaigns and improved diagnosis rates further enhance treatment adoption rates, thereby expanding market scope. Lastly, favorable reimbursement policies in key regions like North America and Europe improve market accessibility and revenue streams, contributing to sustainable market growth.
Dravet Syndrome Treatment Market Development
In January 2025, the FDA granted Relutrigine (PRAX-562) the Rare Pediatric Disease Designation for Dravet syndrome, adding to previous designations for SCN2A-DEE and SCN8A-DEE. This designation is for the drug developed by Praxis Precision Medicines, a small molecule that inhibits persistent sodium currents to control seizures.
In December 2024, Stoke Therapeutics received Breakthrough Therapy Designation from the U.S. FDA for zorevunersen (STK-001), its antisense oligonucleotide therapy for Dravet syndrome in patients with confirmed SCN1A mutations (excluding gain-of-function mutations). Clinical data from Phase 1/2a and open-label extension studies showed substantial and sustained reduction in seizure frequency, plus continuous improvement in cognition and behavior beyond standard anti-seizure drugs.
Key Players
Leading Companies of the Market
Zogenix, Inc.
GW Pharmaceuticals (a subsidiary of Jazz Pharmaceuticals)
Marinus Pharmaceuticals, Inc.
Stoke Therapeutics
UCB Pharma
Lundbeck
Aquestive Therapeutics
Zynerba Pharmaceuticals
Amneal Pharmaceuticals
Innovus Pharmaceuticals
Neurelis, Inc.
Several market players have adopted product innovation and strategic partnerships to expand their footprint. For instance, GW Pharmaceuticals’ successful FDA approval of its Epidiolex product in 2024 significantly enhanced its market revenue and share. Similarly, Zogenix’s acquisition of Stoke Therapeutics in early 2025 enabled a robust gene therapy pipeline enhancement, leading to increased investor confidence and business growth.
Dravet Syndrome Treatment Market Future Outlook
The future outlook for Dravet syndrome treatment is strongly defined by the transition from symptomatic management to disease-modifying therapies. Ongoing clinical trials for gene therapy, antisense oligonucleotides, and mRNA-based treatments are poised to directly target SCN1A mutations—the genetic root cause of the disease. Precision medicine approaches will continue to expand, supported by advances in genomic sequencing and personalized drug design. Digital health platforms and seizure monitoring technologies will also improve real-time management and patient safety. Regulatory agencies and patient advocacy groups are increasingly facilitating faster approval pathways for innovative therapies, suggesting a new era of tailored, life-changing treatments for Dravet patients in the coming years.
Dravet Syndrome Treatment Market Historical Analysis
Historically, the management of Dravet syndrome has been challenging due to its resistance to most conventional antiepileptic drugs. For decades, treatment primarily focused on controlling frequent and prolonged seizures using broad-spectrum anticonvulsants such as valproate, clobazam, and topiramate. However, patients often experienced limited benefits and significant side effects. Over time, the medical community began emphasizing combination therapy and the avoidance of sodium-channel blockers that exacerbate symptoms. The introduction of stiripentol, cannabidiol (Epidiolex), and fenfluramine represented major milestones, offering targeted and safer seizure control. Parallel to pharmacological progress, multidisciplinary interventions, including ketogenic diets, behavioral therapy, and supportive care, have become integral parts of treatment. Advancements in genetic diagnostics have also allowed for earlier and more accurate detection, improving individualized care.
Sources
Primary Research interviews:
Neurologists
Pediatric Epileptologists
Clinical Pharmacologists
Rare Disease Researchers
Databases:
National Institute of Neurological Disorders and Stroke (NINDS)
FDA Orphan Drug Database
Magazines:
Epilepsy Today
Neurology Live
Rare Disease Report
Pharmaceutical Technology
Journals:
Epilepsia
The Lancet Neurology
Journal of Child Neurology
CNS Drugs
Newspapers:
The New York Times (Health)
The Guardian (Science)
The Economic Times (Healthcare)
The Hindu (Health & Research)
Associations:
Epilepsy Foundation
Dravet Syndrome Foundation
International League Against Epilepsy (ILAE)
American Academy of Neurology (AAN)
Share
Share
About Author
Ghanshyam Shrivastava - With over 20 years of experience in the management consulting and research, Ghanshyam Shrivastava serves as a Principal Consultant, bringing extensive expertise in biologics and biosimilars. His primary expertise lies in areas such as market entry and expansion strategy, competitive intelligence, and strategic transformation across diversified portfolio of various drugs used for different therapeutic category and APIs. He excels at identifying key challenges faced by clients and providing robust solutions to enhance their strategic decision-making capabilities. His comprehensive understanding of the market ensures valuable contributions to research reports and business decisions.
Ghanshyam is a sought-after speaker at industry conferences and contributes to various publications on pharma industry.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients