Digital Breast Tomosynthesis Equipment Market is estimated to be valued at USD 3,186.4 Mn in 2025 and is expected to reach USD 8,579.6 Mn in 2032, exhibiting a compound annual growth rate (CAGR) of 15.2% from 2025 to 2032.
Analysts’ Views on Global Digital Breast Tomosynthesis Equipment Market:
Over the forecast period, the global market for digital breast tomosynthesis equipment is anticipated to rise due to the rising incidence of breast cancer and the adoption of cutting-edge imaging technologies. For instance, in October 2022, The American College of Radiology, a professional medical society (ACR) had launched the Contrast-Enhanced Mammography Imaging Screening Trial (CMIST) in collaboration with the Breast Cancer Research Foundation (BCRF) and GE Healthcare. The trial will determine whether contrast-enhanced mammography improves breast cancer detection and reduces false-positive exams in women with dense breasts. The CMIST study seeks to determine if contrast-enhanced mammography (CEM) provides more accurate cancer detection compared to digital breast tomosynthesis (DBT) in women with dense breasts.
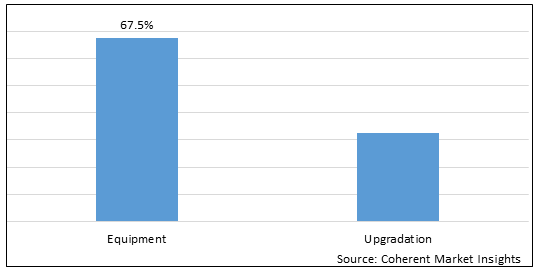
Figure 1. Global Digital Breast Tomosynthesis Equipment Market Share (%), by Product Type, 2025
To learn more about this report, Download Free Sample
Global Digital Breast Tomosynthesis Equipment Market – Driver
Technological advancements and increasing incidence of breast cancer
The market is expanding due to technological developments in digital breast tomosynthesis equipment. Manufacturers are putting their efforts into creating cutting-edge technology, which is enhancing the effectiveness of breast cancer diagnosis and treatment. Examples of this equipment include automated breast ultrasonography systems and biopsy devices. For instance, in December 2022, MedCognetics, Inc., an advanced AI software platform announced it had received U.S Food and Drug Administration (FDA) 510(k) clearance of its artificial intelligence (AI)-enabled software for breast cancer screening, QmTRIAGE. The Company is developing the next generation of AI medical imaging technology with an initial focus on improving outcomes of early breast cancer detection, particularly across a diverse group of patients, as well as helping radiologists with unmanageable caseloads.
Growing awareness about breast cancer screening
Delayed in the breast cancer screening due to pandemic led to a decrease in the breast cancer screening which result’s to a growth in breast cancer after early stages.
For instance, in August 2020, Hologic, Inc., an innovative medical technology company, launched the Back to screening campaign encouraging women to schedule their annual mammograms now that healthcare facilities across the nation are re-opening their doors following closures due to the COVID-19 pandemic.
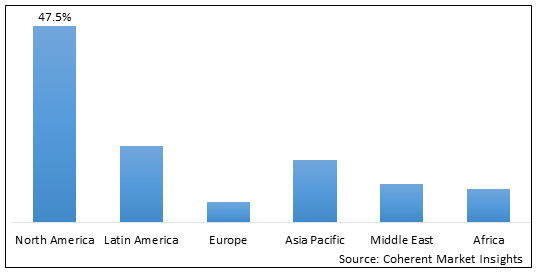
Figure 2. Global Digital Breast Tomosynthesis Equipment Market Value (US$ Million), by Region, 2025
To learn more about this report, Download Free Sample
Global Digital Breast Tomosynthesis Equipment Market- Regional Analysis
Among regions, North America is estimated to hold a dominant position in the global digital breast tomosynthesis equipment market over the forecast period. North America is estimated to hold 47.5% of the market share in 2025. The North America breast tomosynthesis equipment market is expected to witness significant growth in the coming years, driven by factors such as the increasing prevalence of breast cancer and technological advancements in the digital breast tomosynthesis equipment. Thus, the rise digital breast tomosynthesis equipment in over the forecast period.
Global Digital Breast Tomosynthesis Equipment Market – Impact of Coronavirus (COVID-19) Pandemic
Since the COVID-19 virus outbreak in December 2019, the disease spread to over 100 countries across the globe, and the World Health Organization declared it a public health emergency on January 30, 2020.
COVID-19 affected the economy in three main ways: by directly affecting the production and demand of drugs, by creating disruptions in distribution channels, and through its financial impact on firms and financial markets. Due to nationwide lockdowns, several countries such as China, India, Saudi Arabia, the U.A.E., Egypt, and others are facing problems with the transportation of drugs from one place to another.
However, the COVID-19 pandemic had a negative impact on the global digital breast tomosynthesis equipment market. For instance, in December 2021, according to an article published in the Current Health Sciences Journal, the study was done to compare the patient’s addressability to breast imaging techniques for diagnosis, and follow-up in the Clinical Emergency County Hospital of Craiova, Romania. As an overall, by comparing both pre-pandemic years included in the study with the pandemic years, obtained an addressability reduced with 37.3% suggesting the possible future delays in diagnosing breast tumors.
Digital Breast Tomosynthesis Equipment Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 3,186.4 Mn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 15.2% | 2032 Value Projection: | USD 8,579.6 Mn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Major players operating in the global digital breast tomosynthesis equipment market include Hologic, Inc., Internazionale Medico Scientifica-S.R.L, GE Healthcare, Siemens Healthineers, Fujifilm Corporation, Planmed OY, Trivitron Healthcare, Varex Imaging Corporation, and Analogic Corporation |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Global Digital Breast Tomosynthesis Equipment Market Segmentation:
The global digital breast tomosynthesis equipment market report is segmented into by Product Type, and By End User and Region.
By Product Type, the market is segmented into equipment and upgradation. Out of which, the equipment segment is expected to hold a dominant position in the global digital breast tomosynthesis equipment market during the forecast period and this is attributed to the technological advancements in the field of digital breast tomosynthesis equipment.
By End User, the market is segmented into hospitals, and diagnostics centers. Out of which, the hospital segment is expected to dominate the market over the forecast period and this is attributed to the increasing prevalence of breast cancer.
Among all the segmentations, the product type segment has the highest potential due to the increasing prevalence of breast cancer along with improvement in the technological advancement of digital breast tomosynthesis equipment over the forecast period. For instance, in August 2020, Whiterabbit.ai, a developer of software using artificial intelligence to detect cancer at the earliest stages, adds automated mammogram reminders to Arterys' breast cancer-spotting AI. The goals of Whiterabbit’s ACT software go hand-in-hand with those of Arterys’ Breast AI, which aims to improve the detection not only of breast cancer but also of an increased risk of developing the disease.
Global Digital Breast Tomosynthesis Equipment Market Cross Sectional Analysis:
Rising incidence of breast cancer in emerging economies is also expected to boost demand for digital breast tomosynthesis equipment in North America region. For instance, in May 2021, Cancer informatics and digital pathology provider, Inspirata, announced that University Hospitals Dorset NHS Foundation Trust will commence an implementation of its Dynamyx digital pathology software, as part of an agreement struck between enterprise partner, Fujifilm UK Limited and the One Dorset Pathology network. The introduction of Dynamyx at One Dorset Pathology represents the first of several new wins for Fujifilm UK using software, and is a direct award from the PathLAKE digital and computational pathology consortium.
Global Digital Breast Tomosynthesis Equipment Market: Key Developments
On March 20 2023, Lunit, Inc., a medical artificial intelligence (AI) software company, announced that its AI solution for 3D Breast Tomosynthesis (DBT) analysis, Lunit INSIGHT DBT, has met the requirements of the CE marking under Europe's latest Medical Device Regulation (MDR). The MDR CE certification is a more stringent requirement for medical devices than the existing Medical Device Directive (MDD), ensuring higher performance and quality standards.
In December 2021, Freenome Holdings, Inc., a privately held biotech company, announced a partnership with Siemens Healthineers to collaborate in multiomics and radiomic breast cancer diagnostics to identify suitable markers for early breast cancer detection through blood to augment existing imaging technologies. The partnership leverages Freenome's expertise in machine learning and multiomics to detect early cancer, utilizing epigenetic, proteomic, genomic, immunologic and other data types to maximize clinical accuracy for future screening tests. This collaboration will allow Freenome and Siemens Healthineers to share their technologies by connecting imaging and clinical data with molecular data to identify new suitable markers of breast cancer that are complementary to those identified using current imaging.
In November 2021, Arterys, Inc., a medical imaging AI platform, announced it signed a global distribution agreement with iCAD, a global medical technology providing innovative cancer detection and therapy solutions. This partnership will expand access to iCAD's industry-leading AI-powered breast health solutions via Arterys' U.S. FDA-cleared and CE-marked MICA platform to its installed base.
In December 2022, GigCapital5, Inc., a publicly traded special purpose acquisition company, and QT Imaging, Inc., a medical imaging company that develops novel products for body imaging, announced that they have entered into a definitive business combination agreement that would result in QT Imaging becoming a publicly listed company subject to closing. Upon closing of the transaction, the combined company will be named QT Imaging Holdings, Inc. and will be traded on the NYSE under the new ticker symbol “QTI”. The goal is to complete the business combination in the first half of 2023.
On January 31 2023, Abdul Latif Jameel IPR Company Limited, operates as a holding company and provides a range of products and services, including the wholesale distribution of vehicles, consumer financing services, and full service advertising announced a new distribution agreement with iSono Health, a medical technology company in San Francisco, USA, to transform breast care with automated imaging and artificial intelligence (AI).
Global Digital Breast Tomosynthesis Equipment Market: Key Trends
Increase in the adoption of digital breast tomosynthesis (DBT) equipment, integration of artificial intelligence, Integration of artificial intelligence and Increase in demand for 3D imaging.
Increase in the adoption of digital breast tomosynthesis (DBT) equipment
The adoption of DBT equipment has been on the rise due to its improved accuracy in breast cancer detection and reduced false positives when compared to traditional mammography.
Advancements in detector technology:
Detector technology has been improving, resulting in higher image resolution, reduced noise, and sharper images. This advancement has led to the development of DBT systems with a wide range of detector sizes and configurations.
Integration of artificial intelligence:
AI is increasingly being integrated into DBT equipment. It helps in the identification and classification of breast lesions, reducing reading time and improving diagnosis accuracy.
Increase in demand for 3D imaging:
There has been an increase in demand for 3D imaging equipment. As a result, the demand for DBT equipment has also increased, given its ability to provide detailed 3D images of the breast tissue.
For instance, in November 2022, IceCure Medical Ltd., a developer of minimally-invasive cryoablation technology, the ProSense System that destroys tumors by freezing, announced the Centers for Medicare & Medicaid Services ("CMS") assigned its proSense breast cancer cryoablation procedures with CPT Category III code 0581T to ambulatory payment classification 5091, Level 1 Breast/Lymphatic Surgery and Related Procedures. This payment assignment for the procedure will go into effect on January 1, 2023, opening the potential for facilities to be paid, on a case-by-case basis, for these procedures subject to the Company's receipt of U.S. Food and Drug Administration ("FDA") marketing authorization of ProSense for breast cancer. A De Novo Classification Request for breast cancer marketing authorization was filed with the FDA for the initial breakthrough indication of early-stage low-risk breast cancer patients at high risk to surgery in October 2022.
Global Digital Breast Tomosynthesis Equipment Market: Restraint
Limited reimbursement policies for breast tomosynthesis and Lack of awareness about the benefits of digital breast tomosynthesis
Because limited reimbursement policies make it difficult for people to receive medical services such as breast tomosynthesis, the market's expansion may be hampered. In developing countries, there are few reimbursement policies for breast tomosynthesis operations. Patients may not be able to afford the expense of this surgery when reimbursement policies are restrictive, which will reduce demand for breast tomosynthesis. This may ultimately result in low rates of technology adoption in underdeveloped nations and slower market expansion. So, government are taking initiatives to provide the maximum reimbursement policies benefits for breast tomosynthesis. During the forecast period, a number of factors, including a lack of public knowledge regarding digital breast tomosynthesis equipment, are anticipated to restrain the market's expansion. For instance, in March 2022, according to an article published in Health Policy journal, the study was to evaluate the association between state DBT coverage mandates and DBT use, prices, and out-of-pocket payments. Although this study focused on the specific case of DBT, insurance coverage mandates are commonly deployed policy strategies intended to improve patient access to a broad range of health services and to protect patients from financial liability.
Global Digital Breast Tomosynthesis Equipment Market - Key Players
Major players operating in the Global Digital Breast Tomosynthesis Equipment Market include Hologic, Inc., Internazionale Medico Scientifica-S.R.L, GE Healthcare, Siemens Healthineers, Fujifilm Corporation, Planmed OY, Trivitron Healthcare, Varex Imaging Corporation, and Analogic Corporation.
Global Digital Breast Tomosynthesis Equipment Market– Definition
To screen for signs and diagnose cases involving breast cancer accurately requires highly specialized equipment known as Digital Breast Tomosynthesis (DBT). Traditional mammograms often produce unremarkable results due solely to their limited ability to provide a two-dimensional view of the breast. However, DBT's capabilities surpass this limitation. The advance imaging technology implemented by DBT allows practitioners to gain advanced 3D perspectives of the breast by taking multiple scans from various angles. While visually indistinct from conventional mammography equipment, one crucial difference lies between the two. DBT incorporates circular arcs that enable its X-ray tube and detector to attain comprehensive results using precise maneuvers throughout the breast's examination process. These images are then reconstructed by a specialized computer algorithm to create a 3D image of the breast tissue. The 3D image allows radiologists to view the breast tissue in thin sections, making it easier to detect cancers that may be hidden behind overlapping breast tissue. DBT equipment is typically used in combination with standard digital mammography for breast cancer screening.
Share
Share
About Author
Manisha Vibhute is a consultant with over 5 years of experience in market research and consulting. With a strong understanding of market dynamics, Manisha assists clients in developing effective market access strategies. She helps medical device companies navigate pricing, reimbursement, and regulatory pathways to ensure successful product launches.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients