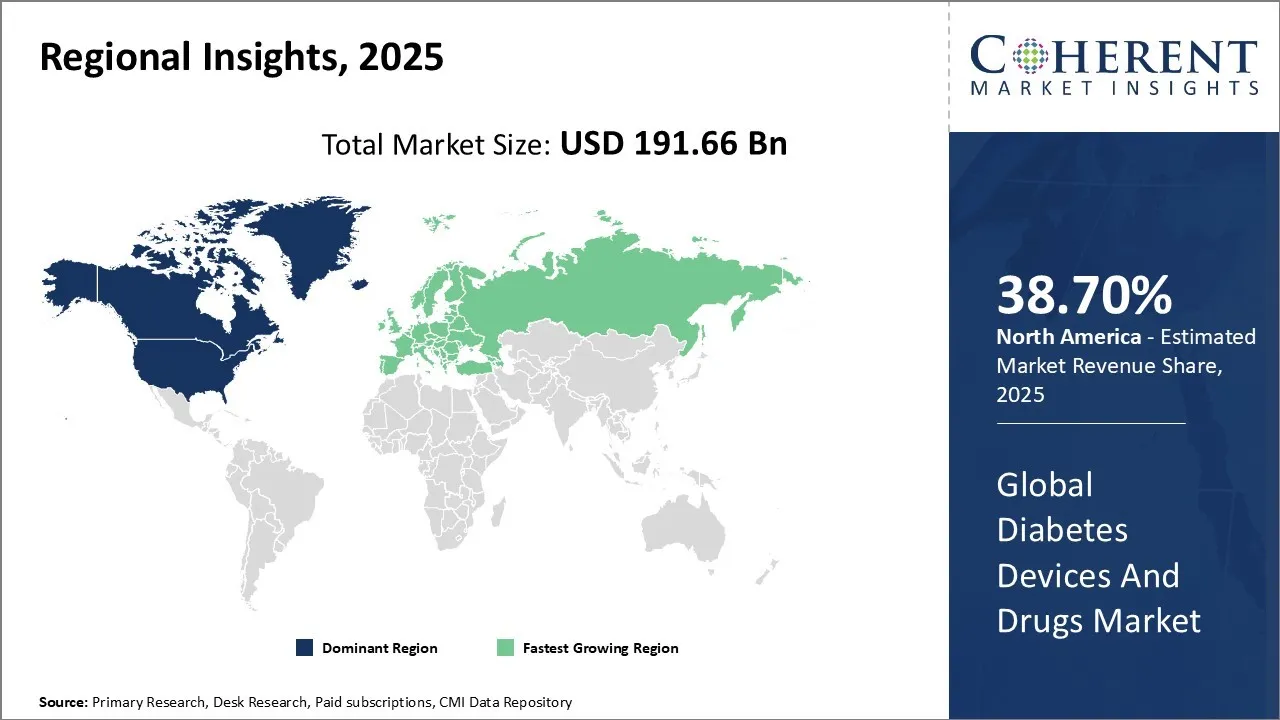
Diabetes Devices And Drugs Market is estimated to be valued at USD 191.66 Bn in 2025 and is expected to reach USD 373.49 Bn in 2032, exhibiting a compound annual growth rate (CAGR) of 10.0% from 2025 to 2032.
The Diabetes Devices and Drugs Market is expanding quickly within the global healthcare sector, fueled by the rising prevalence of diabetes and the growing need for more effective and innovative treatment options. Diabetes, a chronic condition that impacts the body's ability to regulate blood sugar, is divided into Type 1 and Type 2 diabetes, with Type 2 accounting for the majority of cases worldwide.
|
Current Events |
Description and its impact |
|
Regulatory and Clinical Guideline Updates |
|
|
Technological Innovation & Regulatory Approvals |
|
|
APAC Market Dynamics |
|
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
AI-powered devices like continuous glucose monitors (CGMs) and smart insulin pumps collect vast amounts of real-time data. AI algorithms analyze this data to provide personalized insights, predict blood sugar trends, and recommend timely adjustments, helping patients manage their condition more effectively.
In June 2025, IBM and Roche developed AI-powered solution designed to simplify daily diabetes management: Roche’s Accu-Chek® SmartGuide Predict app. This launch highlights the power of cross-industry collaboration and teamwork in enhancing support for people with diabetes and advancing healthcare delivery.
Home blood glucose monitors (BGMs) and continuous glucose monitors (CGMs) are reimbursable under the Durable Medical Equipment (DME) benefit as defined by the Social Security Act (§1861(s)(6)). For beneficiaries to qualify for reimbursement, their DME must meet the reasonable and necessary (R&N) criteria specified in the relevant Local Coverage Determination (LCD). Additionally, specific statutory payment policy requirements must be satisfied.
Medicare expanded coverage to include therapeutic (non-adjunctive) CGM devices under the DME benefit. These devices were officially classified as therapeutic CGMs under CMS Ruling 1682R, distinguishing them from adjunctive models.
In Canada, progress toward universal pharmacare continues. In February 2024, the Minister of Health introduced Bill C-64, An Act respecting pharmacare, marking a key step in the government’s plan to implement national, single-payer coverage for a broad range of diabetes medications. This initiative will be developed in partnership with willing provinces and territories (PTs), aiming to improve access and affordability for diabetes treatments across the country.
The increasing demand for accurate, real-time management of blood glucose levels drives the need for Diagnosis and Monitoring in the Diabetes Devices and Drugs Market. As diabetes, especially Type 2 diabetes, becomes more prevalent, the need for advanced diagnostic tools like continuous glucose monitors (CGMs) and blood glucose meters grows. These devices track glucose fluctuations, allowing patients and healthcare providers to adjust treatment plans promptly. The rising focus on early diagnosis and preventive care, coupled with technological advancements in monitoring systems, further boosts market demand. In May 2023, Roche Diabetes Care India (RDC India) began manufacturing the "Accu-Chek Active" blood glucose monitoring device in India. Sanmina-SCI India produces the product at its Chennai facility, and Parekh Integrated Services distributes it.
The rising prevalence of Type 2 diabetes drives significant demand for treatment and monitoring solutions in the Diabetes Devices and Drugs Market. Factors like rising obesity rates, sedentary lifestyles, and unhealthy diets contribute to the growing incidence of Type 2 diabetes, especially in developed countries. This increase in cases creates a need for more advanced diagnostic tools, medications, and management devices, such as insulin pumps, oral drugs, and continuous glucose monitors (CGMs). As awareness grows and early detection gains more focus, the market is set to expand rapidly.
For instance, in March 2025, Sun Pharmaceutical, planned to launch its experimental anti-obesity and type 2 diabetes drug within the next four to five years, according to Managing Director Dilip Shanghvi's statement on Friday.
To learn more about this report, Download Free Sample
North America dominates the overall market with an estimated share of 38.7%, its growing rapidly due to the rising prevalence of Type 2 diabetes, increasing awareness, and technological advancements. The demand for continuous glucose monitors (CGMs), insulin pumps, and smart insulin pens is rising, while innovations in diabetes medications like SGLT-2 inhibitors and GLP-1 receptor agonists are gaining popularity. The U.S. dominates the market, benefiting from a robust healthcare infrastructure, government support, and favorable reimbursement policies, which continue to drive the region’s market share and expansion. Apart from this, the growing technological innovations is also acting as one of the major factors adding to the diabetes devices and drugs market share. In November 2024, Sol-Millennium® Medical Group launched the InsuJet™ Needle-free Device, a new insulin delivery system for diabetic patients. This innovative technology aims to improve patients’ quality of life by eliminating the pain of traditional needle injections.
The Asia-Pacific Diabetes Devices and Drugs Market is growing rapidly due to the rising incidence of diabetes, particularly in countries like China and India. Urbanization, lifestyle changes, and an aging population are driving the increased demand for diabetes management solutions, including continuous glucose monitors (CGMs), and oral medications. The region's market share is expanding, with Japan and South Korea leading the way in adopting advanced technologies. For instance, in February 2024, Eli Lilly introduced Mounjaro, its diabetes drug and widely popular obesity treatment, in India.
The United States Diabetes Devices and Drugs Market continues to lead globally, driven by the high prevalence of Type 2 diabetes, technological innovations, and strong healthcare infrastructure. In addition, the market is seeing increased adoption of new drug classes like GLP-1 receptor agonists and SGLT-2 inhibitors. The U.S. holds the largest market share, benefiting from favorable reimbursement policies, government healthcare programs, and widespread awareness about diabetes management, making it a key driver of global market trends.
For Instance, in April 2025, Meitheal Pharmaceuticals, Inc., a fully integrated biopharmaceutical company based in Chicago, announced today that it has received U.S. Food and Drug Administration (FDA) approval and launched its liraglutide injection (18mg/3mL), the generic equivalent of Victoza®2, in the U.S.
The rising prevalence of Type 2 diabetes in India, driven by changing lifestyles, urbanization, and an aging population, is fueling growth in the diabetes devices and drugs market. India’s rapid economic development, improving healthcare system, and increasing availability of affordable medications and devices are driving the market forward. While the country’s market share remains smaller compared to Western nations, government initiatives and a growing health-conscious population are expected to further accelerate market expansion in the coming years.
For instance, in March 2025, Morepen Laboratories Ltd. introduced a new medication for chronic kidney disease, heart failure, and Type 2 diabetes. The company stated that Empamore offers millions of patients in India a high-quality, affordable alternative to existing treatments.
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 191.66 Bn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 10.0% | 2032 Value Projection: | USD 373.49 Bn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Abbott Laboratories, Bristol-Myers Squibb, DexCom, Inc., Eli Lilly and Company, Companion Medical, GlaxoSmithKline plc, Glenmark Pharmaceuticals, Johnson & Johnson, Lupin Ltd., F. Hoffmann-La Roche AG, DreaMed Diabetes, Hua Medicine and Sanofi. |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Government initiatives have been fuelling the research and development in the field of diabetes management significantly. This is expected to drive the diabetes devices and drugs market growth over the forecast period. For instance, in March 2021, the Government of Canada announced a new partnership with the Netherlands to fund type 2 diabetes research. This initiative commemorated the 100th anniversary of the discovery of insulin and invests in new research that will support the development of new preventive and therapeutic approaches to reverse the upward trajectory of diabetes prevalence and associated morbidities and reduce the impact of diabetes on individuals, families, and communities. This is further accelerating the diabetes devices and drugs market share.
An important opportunity in the market is the development of personalized treatment plans and innovative diabetes medications. Advances in genomics and patient-specific data are driving the shift towards more tailored drug therapies. GLP-1 receptor agonists and SGLT-2 inhibitors are gaining popularity as they effectively control glucose levels while addressing common comorbidities in diabetic patients, such as cardiovascular diseases and weight management. These drugs are increasingly seen as beneficial for providing more comprehensive care, improving patient outcomes, and reducing complications associated with diabetes.
Share
Share
About Author
Vipul Patil is a dynamic management consultant with 6 years of dedicated experience in the pharmaceutical industry. Known for his analytical acumen and strategic insight, Vipul has successfully partnered with pharmaceutical companies to enhance operational efficiency, cross broader expansion, and navigate the complexities of distribution in markets with high revenue potential.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients