Depression Therapeutics Market is estimated to be valued at USD 14.09 Bn in 2025 and is expected to reach USD 19.17 Bn in 2032, exhibiting a compound annual growth rate (CAGR) of 4.5% from 2025 to 2032.
Analysts’ Views on Global Depression Therapeutics Market:
Market players are focused on the research and development of new drugs in the market, which is expected to drive growth of the global depression therapeutics market. For instance, in December 2022, Otsuka Pharmaceutical Development & Commercialization, Inc., a pharmaceutical company, and Sunovion Pharmaceuticals, Inc., a pharmaceutical company, announced that the first patient has been enrolled in a Phase 2/3 clinical study to evaluate ulotaront (SEP-363856), a trace amine-associated receptor 1 (TAAR1) agonist with 5HT agonist activity, as an adjunctive therapy in the treatment of adults living with major depressive disorder (MDD). Ulotaront, which is also being evaluated in Phase 3 clinical development for the treatment of schizophrenia, is the first TAAR1 agonist to be studied as an adjunctive therapy in the treatment of MDD.
Figure 1. Global Depression Therapeutics Market Share (%), By Drug Type, 2025
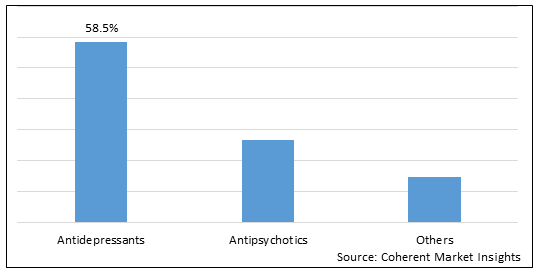
To learn more about this report, Download Free Sample
Global Depression Therapeutics Market– Drivers
Similarly, in May 2022, the Child Mind Institute, the leading independent nonprofit in children’s mental health, launched Dare to Share, a powerful mental health awareness campaign to reduce stigma and normalize conversations about emotional, social, and psychological wellbeing among youth. The campaign was launched in conjunction with Mental Health Awareness Month.
Figure 2. Global Depression Therapeutics Market Share (%), By Region, 2025
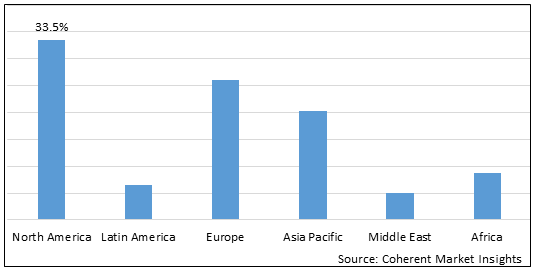
To learn more about this report, Download Free Sample
Global Depression Therapeutics Market- Regional Analysis
Among regions, North America is estimated to hold a dominant position in the global depression therapeutics market over the forecast period. North America is estimated to hold 33.5% of the market share in 2025. The global depression therapeutics market is expected to witness significant growth in the coming years, driven by the high prevalence of mental health disorders, favorable health reimbursement, and increased awareness. Increasing drug approvals by regulatory bodies is contributing to the growth of the depression therapeutics market in the North America region. For instance, in August 2020, The Janssen Pharmaceutical Companies, a subsidiary of Johnson & Johnson announced that the U.S. Food and Drug Administration (FDA) has approved the supplemental new drug application (sNDA) for SPRAVATO (esketamine) CIII nasal spray, taken with an oral antidepressant, to treat depressive symptoms in adults with major depressive disorder (MDD) with acute suicidal ideation or behavior. SPRAVATO is the first and only approved medicine that has been shown to reduce depressive symptoms within 24 hours, providing a new option for significant symptom relief until a longer-term, comprehensive treatment plan can take effect.
Global Depression Therapeutics Market– Impact of Coronavirus (COVID-19) Pandemic
Since the COVID-19 virus outbreak in December 2019, the disease has spread to over 100 countries across the globe, and the World Health Organization declared it a public health emergency on January 30, 2020.
COVID-19 affected the economy in three main ways: by directly affecting the production and demand of drugs, by creating disruptions in distribution channels, and through its financial impact on firms and financial markets. Due to nationwide lockdowns, several countries, such as China, India, Saudi Arabia, the U.A.E., Egypt, and others, are facing problems with the transportation of things from one place to another.
Depression Therapeutics Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 14.09 Bn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 4.5% | 2032 Value Projection: | USD 19.17 Bn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Johnson & Johnson, GlaxoSmithKline plc., Otsuka Pharmaceutical Co., Ltd., Takeda Pharmaceutical Company Limited., Eli Lilly and Company, Sanofi S.A., Pfizer, Inc., Allergan plc., H. Lundbeck A/S, Alkermes, Allergan, Novartis AG, Biogen, Sage Therapeutics, Inc., and AbbVie Inc. |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Global Depression Therapeutics Market Segmentation:
The global depression therapeutics market report is segmented by drug type, by indication, by distribution channel and by region.
Global Global Depression Therapeutics Market- Cross Sectional Analysis:
Key players are gaining drug approvals from regulatory bodies, which is also expected to boost demand for depression therapeutics market in the North America region. For instance, on August 18, 2023, Neurocrine Biosciences, Inc., a Biotech company, announced the U.S. Food and Drug Administration (FDA) has approved INGREZZA (valbenazine) capsules for the treatment of adults with chorea associated with Huntington's disease (HD). INGREZZA is the only selective vesicular monoamine transporter 2 (VMAT2) inhibitor that offers an effective starting dosage that can be adjusted by a patient's healthcare provider based on response and tolerability, with no complex titration. Only INGREZZA offers simple dosing, which is always one capsule, once daily.
Global Depression Therapeutics Market: Key Developments
Global Depression Therapeutics Market: Key Trends
Global Depression Therapeutics Market: Restrain
Global Depression Therapeutics Market - Key Players
Major players operating in the global depression therapeutics market include Johnson & Johnson, GlaxoSmithKline plc., Otsuka Pharmaceutical Co., Ltd., Takeda Pharmaceutical Company Limited., Eli Lilly and Company, Sanofi S.A., Pfizer, Inc., Allergan plc., H. Lundbeck A/S, Alkermes, Allergan, Novartis AG, Biogen, Sage Therapeutics, Inc., and AbbVie Inc.
*Definition: Depression therapeutics medication may be prescribed, along with psychological treatments, when a person experiences a moderate-to-severe episode of depression. Depression is common and can affect people of all ages; however, generally, medication is not recommended as the first choice for the treatment of depression in children and young people. There are many different types of antidepressant medication that have been shown to work, but their effectiveness differs from person to person.
Share
Share
About Author
Vipul Patil is a dynamic management consultant with 6 years of dedicated experience in the pharmaceutical industry. Known for his analytical acumen and strategic insight, Vipul has successfully partnered with pharmaceutical companies to enhance operational efficiency, cross broader expansion, and navigate the complexities of distribution in markets with high revenue potential.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients