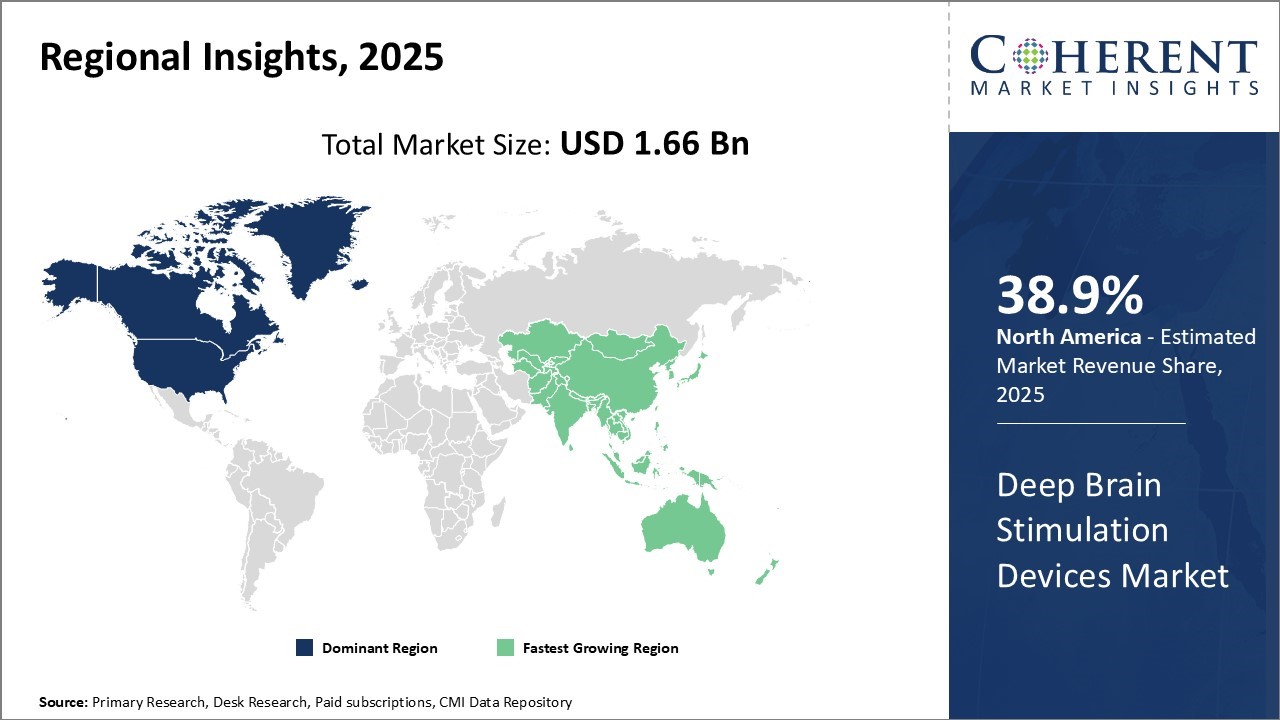
The deep brain stimulation devices market is estimated to be valued at USD 1.66 Bn in 2025 and is expected to reach USD 3.38 Bn by 2032, exhibiting a compound annual growth rate (CAGR) of 10.7% from 2025 to 2032.
The deep brain stimulation devices market is witnessing positive trends with major players continuously innovating and launching new products with enhanced features. Technological advancements have allowed improved targeting abilities and battery lives in these devices. This is anticipated to boost the adoption of deep brain stimulation especially for neurological conditions.
However, the high cost of Deep Brain Stimulation (DBS) therapy, coupled with the complexity and potential risks associated with DBS implantation, is expected to pose challenges to the growth of the deep brain stimulation devices, deep brain stimulation devices market.
|
Current Events |
Description and its impact |
|
Clinical Trial Advancements for New Indications |
|
|
Market Consolidation and Competitive Shifts |
|
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
The global demographic shift toward an aging population is fundamentally reshaping the healthcare landscape, with profound implications for the Deep Brain Stimulation (DBS) devices market. As populations age, the prevalence of neurological disorders that benefit from DBS therapy increases dramatically, creating substantial demand for these sophisticated medical devices.
For instance, according to a report by the World Health Organization (WHO), by 2030, 1 in 6 people in the world will be aged 60 years or over. At this time the share of the population aged 60 years and over will increase from 1 billion in 2020 to 1.4 billion, underscoring the growing need for targeted neurological interventions like DBS.
In terms of Product Type, Single-channel DBS Devices segment is estimated to contribute the highest market share with 52.6% in 2025, owing to their affordable price point and proven effectiveness.
As hospitals, clinics, and other healthcare providers aim to treat more patients while managing costs, single-channel devices have emerged as an attractive option. Their simpler, single lead design allows for lower production expenses which are passed on to customers. This has made deep brain stimulation therapy accessible to a wider population grappling with neurological conditions.
In terms of Application, Pain Management segment is estimated to contribute the highest market share of 36.7% in 2025. Though Deep Brain Stimulation was first approved to treat movement disorders like Parkinson's disease, its use has rapidly expanded into new therapeutic areas.
Chronic pain affects millions and poses a major global health challenge due to limited treatment options and side effects of medication. As a result, neurological centers have actively evaluated deep brain stimulation as an alternative approach for pain syndromes. Studies show DBS can significantly reduce intensity and interference of various types of pain including those related to trauma, arthritis, and neurological damage.
In terms of End User, Hospitals segment is estimated to contribute the highest market share of 40.6% in 2025, owing to their advanced infrastructure and multidisciplinary teams.
Deep brain stimulation requires sophisticated operating rooms, specialized imaging equipment, and coordinated care by neurosurgeons, neurologists, nurses, and technicians. It also demands comprehensive follow-up support for optimal therapeutic effect. While some clinics have capability for DBS, hospitals are best equipped to cater to the entire integrated process from patient selection to long-term management.
Artificial Intelligence (AI) is reshaping the landscape of the Deep Brain Stimulation (DBS) devices market by enhancing the precision, adaptability, and overall effectiveness of neurological therapies.
To learn more about this report, Download Free Sample
North America dominates the deep brain stimulation devices market with an estimated share of 38.9% in 2025. This can be attributed to factors such as the presence of major industry players, availability of reimbursements, growing aging population susceptible to neurological disorders, and technical advancements.
In May 2025, the 77th American Academy of Neurology (AAN) Annual meeting that took place in San Diego, CA, and online, brought together neurologists and neuroscience professionals to discuss the latest advances in the understanding and treatment of a variety of neurological disorders. With over 14,500 experts in neurological health from 110 countries and all 50 U.S. states, the AAN 2025 Annual Meeting boasted the best of this expertise. The gathering transcended borders to expand, build on, and strengthen knowledge in research and clinical practice.
The Asia Pacific region exhibits the fastest growth with an estimated share of 28.2% in 2025 and is expected to rise at a considerable rate over the forecast period. This can be credited to the improving healthcare infrastructure, increasing medical tourism, growing prevalence of neurological disorders due to changing lifestyle habits, rising disposable incomes, and supportive government policies focused on strengthening the medical devices industry across countries in the region.
For instance, as per a report by the Global Wellness Institute, among the world’s top 25 wellness markets, Thailand ranked #1 in wellness market growth from 2022 to 2023, with 28.4% growth, according to GWI’s latest Wellness Economy Monitor. Thailand’s wellness market grew from USD 31.6 billion in 2022 to USD 40.5 billion in 2023. Globally, Thailand is ranked as the 24th largest wellness economy, 9th in the Asia-Pacific region, and 15th worldwide for wellness tourism.
The U.S. deep brain stimulation devices market is anticipated to lead globally during the forecast period, driven by the rising prevalence of neurological disorders such as Parkinson's disease, dystonia, and essential tremors, along with an increasing number of clinical trials targeting psychiatric disorders. Technological advancements in DBS, including improved lead designs, implantable pulse generators, and remote monitoring capabilities, are enhancing the therapy's effectiveness and safety in the U.S.
In February 2025, the U.S. Food and Drug Administration approved the first adaptive, or self-adjusting, deep brain stimulation (DBS) system for Parkinson’s — the Medtronic Percept.
The deep brain stimulation devices market in Japan is experiencing substantial growth, fueled by the rising demand for innovative treatments for neurological disorders. DBS is approved in Japan for treating Parkinson's disease, and the market has shown steady expansion in recent years. The increasing prevalence of neurological disorders, driven by the country's aging population, has significantly boosted the demand for DBS devices.
Germany's robust healthcare system supports the demand for deep brain stimulation devices, with significant investments in medical technology. Boston Scientific Corporation has received CE Mark and launched the fourth-generation vercise genus deep brain stimulation (DBS) system in Germany. The system, featuring full-body MRI compatibility and Bluetooth capabilities, is designed to treat Parkinson's disease, essential tremor, and dystonia through precise brain stimulation for optimal symptom relief.
In January 2025, Medtronic achieved CE Mark approval for BrainSense adaptive deep brain stimulation and Electrode Identifier, a groundbreaking advance in personalized, sensing-enabled care for people with Parkinson's through innovative brain-computer interface technology.
The U.K.'s National Health Service (NHS) has been integrating advanced deep brain stimulation technologies to address neurological disorders. In November 2022, the NHS adopted new DBS systems with enhanced MRI compatibility, improving treatment accessibility and patient safety. These developments highlight the global expansion and acceptance of DBS devices, driven by technological innovations and supportive healthcare policies.
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 1.66 Bn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 10.7% | 2032 Value Projection: | USD 3.38 Bn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Abbott, Medtronic, Boston Scientific Corporation, Neuropace Inc., LivaNova Plc, Renishaw Plc, Aleva Neurotherapeutics S.A., Beijing Pinchi Medical Equipment Co. Ltd, Nexstim, St. Jude Medical (now part of Abbott), Cyberonics (now part of LivaNova), EBR Systems, IntraPace, Nevro Corp, and Stimwave Technologies |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
*Definition: The Deep Brain Stimulation (DBS) Devices Market refers to the global industry involved in the research, development, production, and commercialization of implantable medical devices used to deliver electrical stimulation to specific areas of the brain. These devices are primarily used to treat neurological and psychiatric disorders, such as Parkinson’s disease, essential tremor, dystonia, epilepsy, and obsessive-compulsive disorder (OCD).
Share
Share
About Author
Manisha Vibhute is a consultant with over 5 years of experience in market research and consulting. With a strong understanding of market dynamics, Manisha assists clients in developing effective market access strategies. She helps medical device companies navigate pricing, reimbursement, and regulatory pathways to ensure successful product launches.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients