Cryptococcosis Treatment Market Size and Forecast – 2026 – 2033
The Cryptococcosis treatment market was valued at about USD 5.76 billion in 2026 and is forecast to reach around USD 10.04 billion by 2033, growing at a CAGR of 7.2 % from 2026 to 2033.
Global Cryptococcosis Treatment Market Overview
Cryptococcosis treatment focuses on managing a serious fungal infection caused by Cryptococcus neoformans or C. gattii, primarily affecting immunocompromised patients. Therapy depends on disease severity and site of infection. Severe or CNS infections are treated with induction therapy using amphotericin B plus flucytosine, followed by consolidation and maintenance with fluconazole. Mild to moderate pulmonary cases may be managed with oral azole antifungals alone. Early diagnosis, prolonged treatment duration, and careful monitoring for drug toxicity are essential. Treatment guidelines emphasize relapse prevention, especially in HIV/AIDS and transplant patients.
Key Takeaways
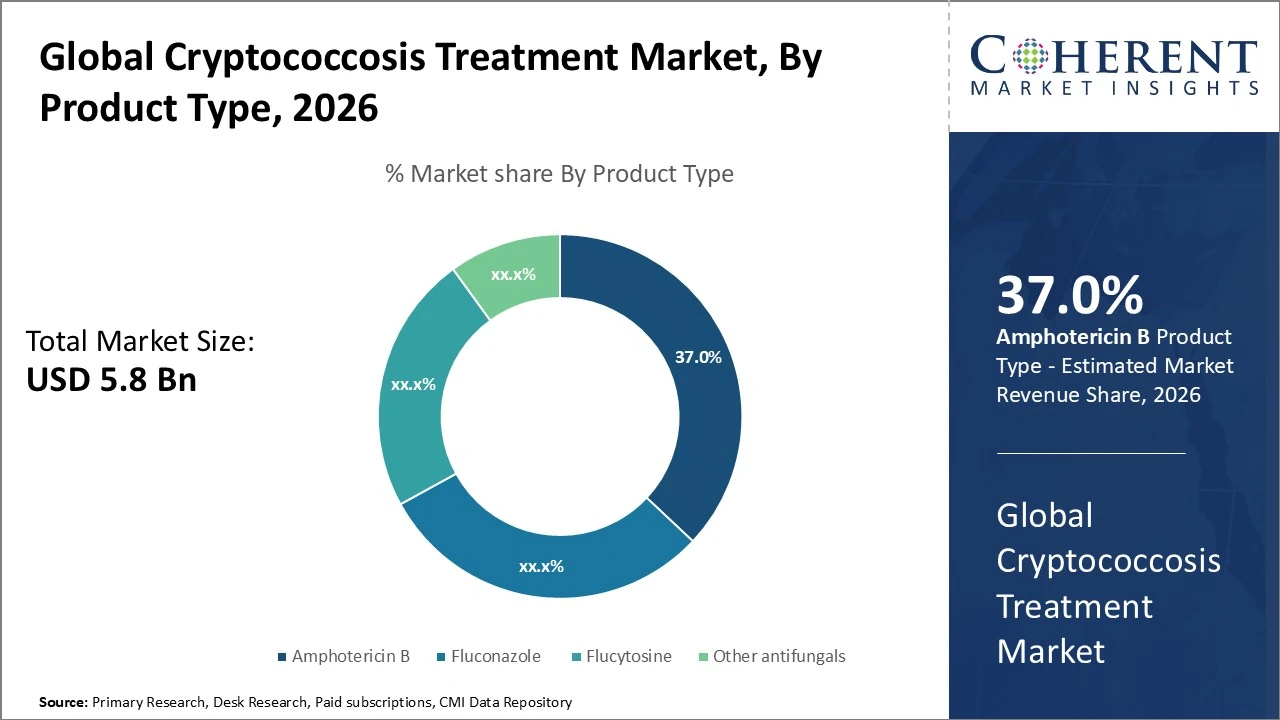
By Product Type- product segments are led by Amphotericin B, holding around 37 % of revenue.
Oral therapies dominate with roughly 52 % share due to ease of use.
Hospitals are the dominant end-user, holding roughly 57% of revenue.
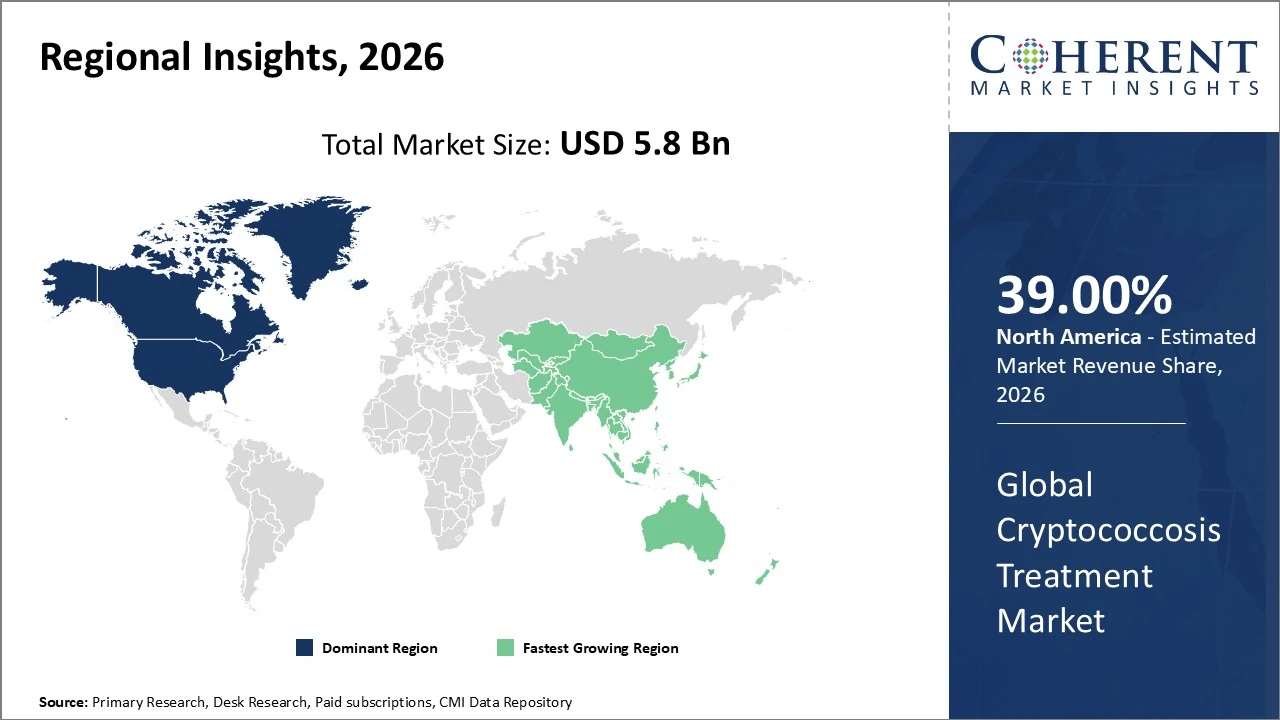
North America dominates the global Cryptococcosis treatment market in 2026 with about 39 % revenue share.
Cryptococcosis Treatment Market Segmentation Analysis
To learn more about this report, Download Free Sample
Cryptococcosis Treatment Market Insights, By Product Type
In the Cryptococcosis treatment market, product segments are led by Amphotericin B, holding around 37 % of revenue due to its strong efficacy in severe cryptococcal infections. Fluconazole follows with roughly 30 % share, driven by its oral use and suitability for maintenance therapy, while Flucytosine accounts for about 23 % as an important combination agent. Other antifungals make up the remaining 10 % of the market. This segmentation reflects continued reliance on established antifungal drugs with broad clinical application and growing demand for safer, accessible therapies.
Cryptococcosis Treatment Market Insights, By Route of Administration
Oral therapies dominate with roughly 52 % share due to ease of use and patient compliance in long-term antifungal treatment. Intravenous administration follows, increasingly adopted for severe or acute cryptococcal infections, and represents a significant portion of remaining market share as hospitals prioritize rapid systemic action. Inhalation and other delivery methods comprise the minor remainder of technology segments, reflecting niche use or emerging administration approaches in clinical settings. These trends highlight patient-centered delivery innovation and clinical preferences.
Cryptococcosis Treatment Market Insights, By End-User
In the global Cryptococcosis treatment market, hospitals dominate end-users with about 57 % share in 2026, driven by inpatient care, advanced diagnostics, and intensive antifungal therapy needs for severe cryptococcal infections. Clinics follow as significant end-users, especially for outpatient initiation of treatment and maintenance therapy, and are projected to grow faster as ambulatory care expands. Homecare settings and others make up the remaining share, reflecting rising preferences for at-home management and supportive services in long-term cryptococcosis care.
Cryptococcosis Treatment Market Trends
Development of new drug formulations (e.g., liposomal amphotericin B) and synergistic combinations (such as amphotericin B + flucytosine) is improving efficacy and reducing side effects, boosting adoption in severe cases.
Advanced diagnostic tools like PCR and lateral flow assays are enabling quicker detection of cryptococcal infections, supporting timely therapy and better outcomes.
Growth in Asia-Pacific and other emerging markets, driven by rising HIV/AIDS prevalence and improved healthcare infrastructure, is expanding treatment availability and market demand.
Cryptococcosis Treatment Market Insights, By Geography
To learn more about this report, Download Free Sample
North America Cryptococcosis Treatment Market Analysis and Trends
North America dominates the global Cryptococcosis treatment market in 2026 with about 39% revenue share, led by the U.S. due to advanced healthcare infrastructure, high antifungal therapy adoption, and significant R&D investments. The region’s strong diagnostic capabilities and widespread access to treatments like amphotericin B and fluconazole support sustained market leadership. Growing incidence of opportunistic infections among immunocompromised populations further fuels demand. North America is expected to maintain steady growth, supported by continued pharmaceutical innovation and healthcare expenditure.
Asia Pacific Cryptococcosis Treatment Market Analysis and Trends
The Asia Pacific Cryptococcosis treatment market holds about 23 % of global share, driven by increasing prevalence of HIV/AIDS and other immunocompromised conditions in populous countries like China and India. Growing healthcare infrastructure and expanding access to antifungal therapies are boosting diagnosis and treatment uptake across the region. APAC is also projected to be the fastest-growing regional market, with high CAGRs supported by rising disease awareness, improved diagnostics, and investment in healthcare services. China leads within APAC due to robust healthcare expansion and large patient base.
Cryptococcosis Treatment Market Outlook for Key Countries
USA Cryptococcosis Treatment Market Analysis and Trends
The U.S. cryptococcosis treatment market is the largest contributor within North America, capturing the majority of the region’s share of 85% due to advanced healthcare infrastructure, high diagnosis rates, and significant use of antifungal therapies like amphotericin B, fluconazole, and voriconazole. Growth is driven by the prevalence of opportunistic fungal infections in immunocompromised populations and strong pharmaceutical R&D. The market exhibits steady expansion supported by government support for infectious disease management, widespread insurance coverage, and ongoing clinical adoption of novel and combination antifungal regimens.
Germany Cryptococcosis Treatment Market Analysis and Trends
The Germany cryptococcosis treatment market is expanding steadily, supported by advanced healthcare services, high clinical awareness of fungal infections, and strong R&D in antifungal therapies. Germany is a key contributor within Europe’s ~29 % regional share of the cryptococcosis treatment market, reflecting robust adoption of effective treatments and early diagnostics. The market is driven by increasing numbers of immunocompromised patients and government investment in infectious disease care. Hospitals and specialty clinics predominantly use combination antifungal regimens, fostering market growth. Ongoing innovation and early drug adoption are expected to sustain a notable CAGR compared to broader European trends.
Analyst Opinion
Established antifungals like amphotericin B and fluconazole together hold about ~67 % of market revenue, reflecting clinician preference and broad usage.
North America commands roughly ~39 % of global market share, supported by advanced healthcare infrastructure and high diagnosis rates.
Asia Pacific is forecasted as the fastest‑growing region, with ~15 %+ CAGR, due to high HIV prevalence and expanding healthcare access.
Analysts emphasize a shift toward combination therapies and novel delivery systems, expected to capture ~10 % of new development focus as personalized antifungal approaches expand.
Market Scope
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2025 | Market Size in 2026: | USD 5.76 billion |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2026 To 2033 |
| Forecast Period 2026 to 2033 CAGR: | 7.2% | 2033 Value Projection: | USD 10.04 billion |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: | Pfizer Inc., Bristol-Myers Squibb, Gilead Sciences, Inc., Merck & Co., Inc., Astellas Pharma Inc., Bayer AG, Asahi Kasei Pharma Corporation | ||
| Growth Drivers: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Cryptococcosis Treatment Market Growth Factors
The global cryptococcosis treatment market is driven by rising incidence of opportunistic fungal infections, especially among HIV/AIDS patients, organ transplant recipients, and immunocompromised populations. Increasing awareness and early diagnosis through advanced diagnostic tools like PCR and lateral flow assays boost treatment adoption. The expansion of healthcare infrastructure in emerging regions, coupled with government initiatives for infectious disease management, fuels market demand. Growth is further supported by ongoing R&D in novel antifungal formulations and combination therapies that improve efficacy and reduce side effects. Rising hospitalizations, aging populations, and accessibility to oral and intravenous antifungal therapies also contribute to sustained market expansion.
Cryptococcosis Treatment Market Development
In April 2025, the not-for-profit Drugs for Neglected Diseases initiative (DNDi) and its partners launched Phase II clinical trials in Malawi and Tanzania for a new sustained-release, patient-friendly formulation of flucytosine to treat cryptococcal meningitis.
Key Players
Leading Companies of the Market
Merck & Co., Inc.
Pfizer Inc.
Gilead Sciences, Inc.
Astellas Pharma Inc.
Bayer AG
Asahi Kasei Pharma Corporation
Key players include Pfizer, Gilead Sciences, Merck & Co., Bristol‑Myers Squibb, Astellas Pharma, Bayer, Bayer AG and Asahi Kasei Pharma corporation. These companies drive market growth through innovative antifungal therapies, combination treatments, global distribution, and R&D in safer, more effective formulations.
Cryptococcosis Treatment Market Future Outlook
The cryptococcosis treatment market is poised for steady growth through 2033, driven by increasing opportunistic infections in immunocompromised populations, rising awareness, and improved diagnostic capabilities. Analysts expect emerging markets, particularly in Asia Pacific and Latin America, to expand rapidly due to enhanced healthcare infrastructure and access to antifungal therapies. Innovation in novel formulations, combination therapies, and targeted delivery systems will improve efficacy and safety, attracting broader adoption. Hospital and clinic-based treatment will continue to dominate, while homecare and outpatient management may rise.
Cryptococcosis Treatment Market Historical Analysis
Historically, the cryptococcosis treatment market has been shaped by the growing prevalence of opportunistic fungal infections, particularly among HIV/AIDS and immunocompromised patients. Early treatment relied primarily on amphotericin B, which, despite efficacy, posed significant toxicity risks. Over time, the introduction of oral azoles like fluconazole and combination therapies with flucytosine improved safety, efficacy, and patient compliance. Market growth was initially concentrated in North America and Europe, where healthcare infrastructure and diagnostic capabilities were advanced. Over the past decade, rising awareness, expanding healthcare access in emerging regions, and continuous R&D in antifungal therapies have driven steady market expansion.
Sources
Primary Research Interviews:
Industry Executives & Decision Makers
Healthcare Professionals
R&D & Clinical Experts
Regulatory Authorities
Databases:
PubMed / Medline
ClinicalTrials.gov
WHO Global Health Observatory
Journals:
Clinical Infectious Diseases (CID)
The Journal of Infectious Diseases (JID)
Mycoses
Medical Mycology
Newspapers:
The Washington Post
Financial Times
The Guardian
The New York Times
Associations:
Infectious Diseases Society of America (IDSA)
American Society for Microbiology (ASM)
European Society of Clinical Microbiology and Infectious Diseases (ESCMID)
International Society for Human and Animal Mycology (ISHAM)
Share
Share
About Author
Vipul Patil is a dynamic management consultant with 6 years of dedicated experience in the pharmaceutical industry. Known for his analytical acumen and strategic insight, Vipul has successfully partnered with pharmaceutical companies to enhance operational efficiency, cross broader expansion, and navigate the complexities of distribution in markets with high revenue potential.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients