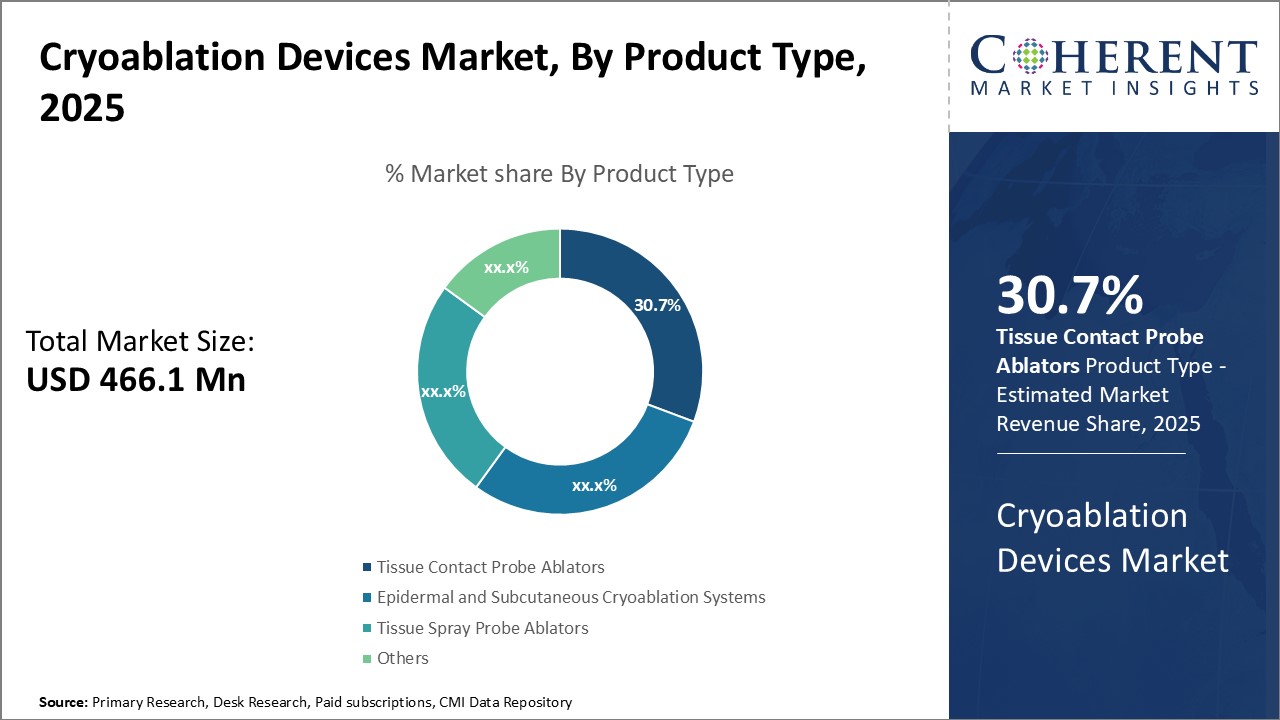
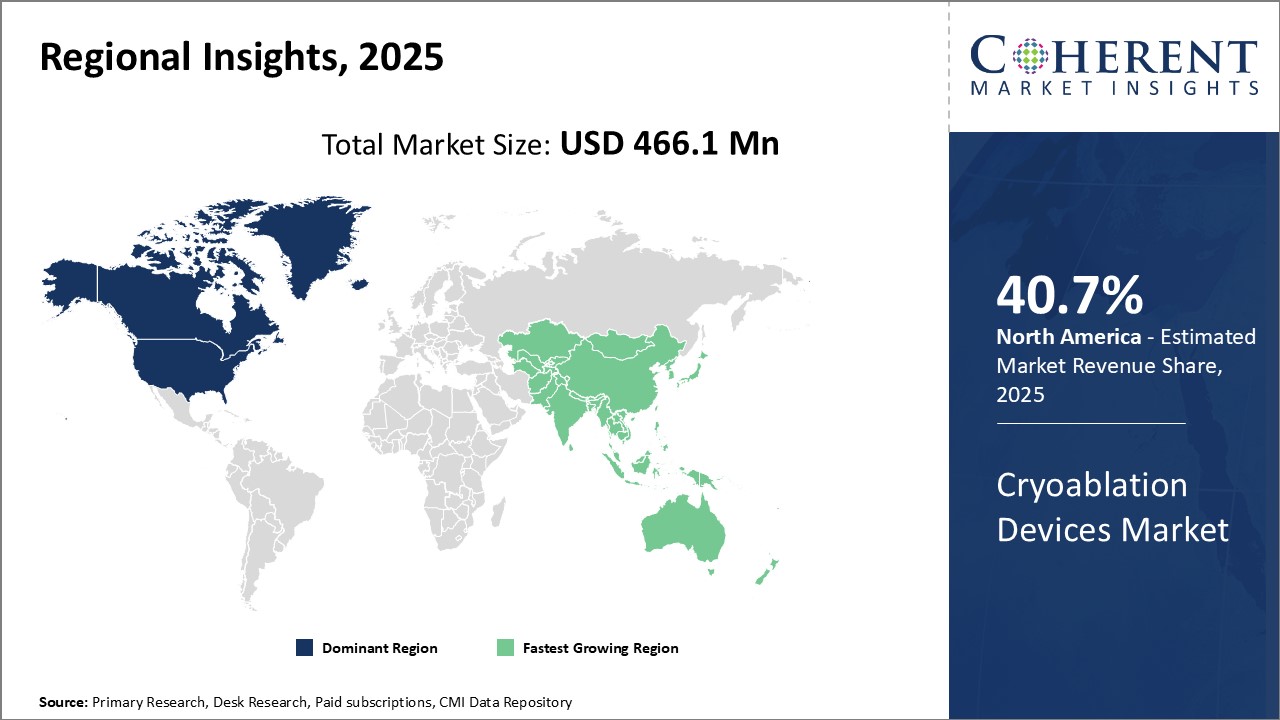
Global cryoablation devices market is estimated to be valued at USD 466.1 Mn in 2025 and is expected to reach USD 1,181.4 Mn by 2032, exhibiting a compound annual growth rate (CAGR) of 14.2% from 2025 to 2032.
Discover market dynamics shaping the industry: Download Free Sample
Rising prevalence of cancer and other cardiovascular diseases, growing geriatric population, and technological advancements in cryoablation devices can drive the market growth. The development of compact, precise, and easy-to-use cryoablation systems have boosted their adoption rate as compared to other conventional ablation procedures. Favorable government policies and rising healthcare expenditures also drives the market growth. However, lack of skilled professionals and limited clinical evidence can hamper the market growth
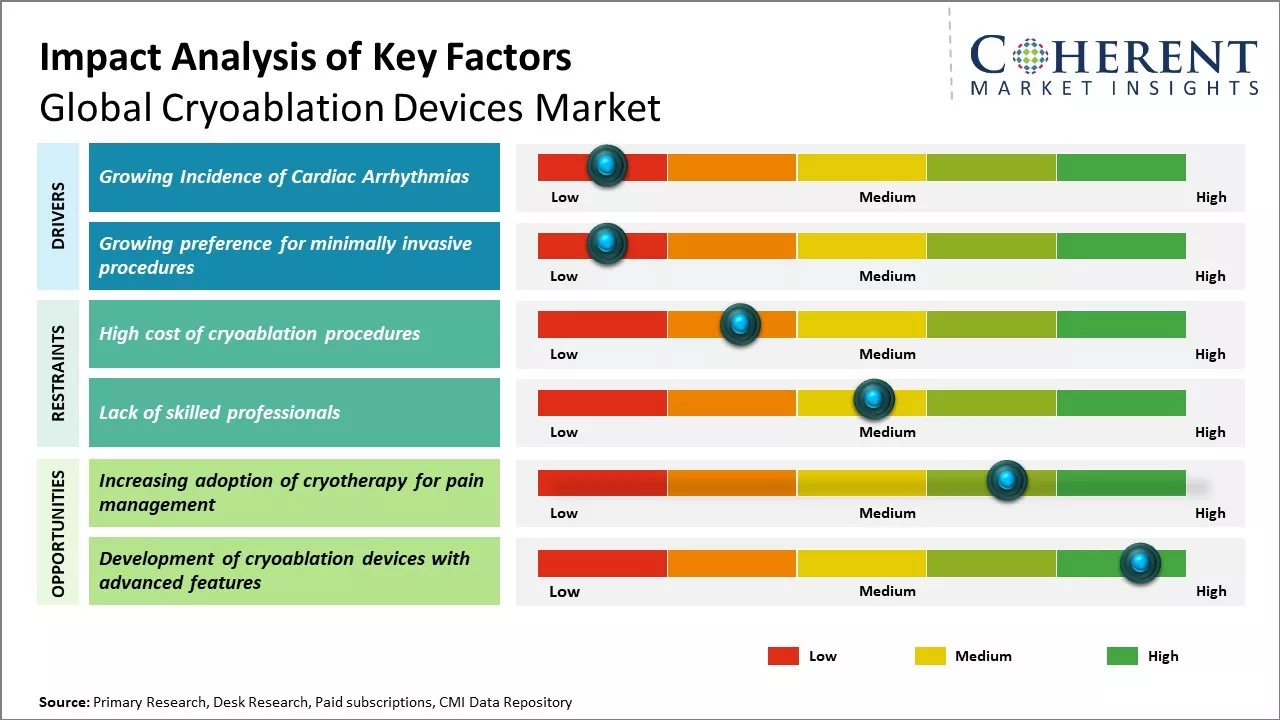
Growing incidence of cardiac arrhythmias
Global cryoablation devices market growth is driven by rising incidence of cardiac arrhythmias worldwide. Cardiac arrhythmias have become highly common these days due to changing lifestyle and increasing stress levels. Prolonged sitting jobs, lack of physical exercise, diet rich in fat and calories leads to irregular heart rhythms. According to some estimates, over 33 million people suffer from irregular heartbeats globally. Atrial fibrillation is the most frequently diagnosed arrhythmia and associated with several complications if left untreated. Cryoablation is gaining popularity as a treatment option for various types of cardiac arrhythmias due to advantages over other ablation methods. The procedure treats arrhythmia by creating frozen non-conducting scars in the heart muscles to block abnormal conduction pathways. It can isolate and deactivate cardiac tissues responsible for irregular heartbeat. Cryoablation offers higher success rates, minimal effects on heart tissues and reduces risks of complications compared to radiofrequency ablation. Rising healthcare expenditure in developed nations has enabled wider acceptance of advanced ablation devices and techniques for rhythm disorders management. For instance, according to British Heart Foundation in May 2023, the number of people diagnosed with atrial fibrillation, a heart rhythm condition increasing the risk of stroke, has surpassed 1.5 million for the first time, marking a 50% rise since 2013. Approximately 1 in 45 people in the U.K. live with this condition. Atrial fibrillation, the most common type of abnormal heart rhythm, contributes to one in five strokes, making those affected five times more likely to experience a severe stroke. Improved awareness and diagnosis have led to better recognition, yet an estimated 270,000 individuals in the U.K. remain undiagnosed. Symptoms like palpitations, breathlessness, and dizziness are common, though some people may not experience any symptoms, complicating early detection and treatment.
Get actionable strategies to beat competition: Download Free Sample
Growing preference for minimally invasive procedures
Growing preference for minimally invasive surgical procedures globally among patients as well as physicians can drive the cryoablation devices market growth. Minimally invasive surgeries are preferred due to benefits such as lesser pain, smaller incisions, shorter hospital stay, quicker recovery time and reduced scarring. Cryoablation, which uses extreme cold to destroy diseased tissue, has emerged as a popular minimally invasive technique for treating cancer and other conditions affecting sites like liver, kidneys and prostate. Cryoablation is often considered as an attractive alternative to traditional surgical procedures for a variety of tumors. Cryoablation of small kidney tumors and early-stage prostate cancer has demonstrated similar clinical outcomes to partial or radical nephrectomy and radical prostatectomy respectively but with considerably lower risks of complications and side effects for patients. As the procedure can be performed via insertion of a probe or catheter through small skin incisions utilizing image guidance, it causes less damage to surrounding healthy tissues as compared to open or laparoscopic surgery. This has led to faster convalescence and return to normal activities for patients. With growing prevalence of cancers worldwide and rising focus on improving quality of life of patients, there has been huge demand for minimally invasive options.
Key Takeaways from Analyst
Global cryoablation devices market growth is driven by factors such as rising geriatric population suffering from cardiac arrhythmias and cancer globally. Furthermore, rising preference for minimally invasive procedures and shorter recovery period associated with cryoablation therapy as compared to traditional surgery can also drive the market growth.
North America currently dominates the market, owing to growing awareness about cryotherapy and established healthcare infrastructure and reimbursement policies in the region. However, Asia Pacific region is expected to offer lucrative opportunities due to growing healthcare expenditure, increasing incidence of cancer, and rapidly expanding medical tourism industry in the region.
Lack of clinical evidence validating cryotherapy for some indications and high costs involved in cryoablation procedures can hamper the market growth. Moreover, limited reimbursement coverage offered for cryoablation therapy in developing regions can also hamper the market growth.
Growing preference for outpatient surgical centers where majority of cryoablation procedures are performed can open new avenues. Ongoing R&D for development of next-generation cryoablation devices with improved features such as integrated imaging, navigation, and robotics can boost adoption of these devices. Market players are also focusing on emerging markets through collaborations to leverage opportunities in these regions.
Market Challenges: High cost of cryoablation procedures
The high cost of cryoablation procedures can hamper the global cryoablation devices market growth. Cryoablation is a minimally invasive procedure that uses extreme cold produced by liquid nitrogen or argon gas to destroy abnormal or diseased tissue. However, the equipment and devices required for cryoablation are highly sophisticated and expensive. Cryoablation devices use state-of-the-art technologies like imaging systems, probes, consoles and other ancillary parts that increases the cost of treatment. The cost of a basic cryoablation procedure range from USD 10,000 to USD 30,000 depending on the organ being treated and complexity of the case. This does not include additional hospital stay, medication and follow up costs. When compared to alternative treatment options like radiofrequency ablation, microwave ablation or surgery, cryoablation is significantly more expensive. This high cost puts cryoablation out of reach for a large section of patients, especially in developing nations with limited access to healthcare and medical insurance. According to World Bank Data, over 1.2 billion people globally lack access to essential health services and almost 100 million people are pushed into extreme poverty due to out-of-pocket healthcare expenses every year.
Market Opportunities: Increasing adoption of cryotherapy for pain management
Increasing adoption of cryotherapy as a pain management method can provide significant opportunities for growth of global cryoablation devices market. Cryotherapy is gaining recognition from both patients and doctors as a drug-free option for relieving pain. It uses cold temperatures to denervate nerve fibers or cause controlled damage to pain-conducting nerves and tissues. This helps reduce sensitivity and block the transmission of pain signals to the brain. Cryotherapy offers several advantages over drug-based pain therapies and other ablation procedures. It produces precisely targeted pain relief with minimal risks of complications compared to treatments like radiofrequency ablation. Cryoablation procedures are generally non-invasive or minimally invasive, allowing for reduced recovery times. This makes the treatment appealing to a growing population of patients seeking natural or drug-free pain interventions. According to the Centers for Disease Control and Prevention, the use of minimally invasive procedures for chronic pain management had increased by 50% between 2000 to 2010 in the U.S.
Discover high revenue pocket segments and roadmap to it: Download Free Sample
By Product Type - Success through Precision and Effectiveness
In terms of product type, tissue contact probe ablators segment is estimated to contribute the highest market share of 30.7% in 2025, due to its precise tissue contact and ablation. Tissue contacts probe ablators work by direct contact of probes with target tissues, allowing for precise placement and localized energy delivery. This ensures effective treatment by annihilating diseased tissues with minimal damage to surrounding healthy tissues. The precise contact and focused energy deposition aid in treatment of difficult to reach tumor sites like those in lungs, liver, and others without requiring surgery. Being minimally invasive, these cause lesser pain and trauma to patients as compared to surgical procedures. The reproducible, localized treatment action and swift recovery help address patient expectation of effective yet less painful treatment options. Continuous product innovation enables treatment of complex tumor configurations with multiple probes in single sitting, improving clinical outcomes. Strong clinical evidence validating safety, efficacy and procedural benefits drives continued patient and physician preference for tissue contact probe ablators over other cryoablation product alternatives.
By Application - Optimizing Clinical Outcomes
In terms of application, lung cancer segment is estimated to contribute the highest market share of 25.6% in 2024, due to enhanced clinical viability of cryoablation in lung malignancies. Cryoablation offers distinct advantages over surgical resection for lung tumors, given anatomical location of lungs and sensitivity of pulmonary system. Minimally invasive cryoablation aids in treatment of early as well as late-stage lung tumors across central, peripheral and subpleural locations with minimal loss of lung functioning. This is vital as lung function significantly impacts clinical outcomes and quality of life post-treatment. Ongoing improvements in cryoablation technologies enable treating previously inoperable tumors, thus, boosting adoption. Cryoablation also allows repeated sessions if required without added risk, thus, optimizing clinical management of lung cancer. The ability to deploy multiple probes per sitting helps more complete ablations even in complex lung tumor configurations. This enhances local tumor control rates and improves survival benefits comparable to other standard therapies. Supported by robust clinical research substantiating outcomes, cryoablation is now considered as mainstay treatment option in lung malignancies.
By End User - A Culture of Care
In terms of end user, hospitals & clinics segment is estimated to contribute the highest market share of, due to their focus on furthering standards of cancer care. Hospitals & clinics consider cryoablation as an important addition to care pathways due to minimally invasive profile, favorable safety over surgery and ability to optimize multidisciplinary management of cancer patients. A significant number of hospitals worldwide have established dedicated cryoablation suites as cryoablation gains traction as a mainstream treatment offering. Their continued investments in procuring latest ablation technologies, training medical personnel and partnering with key opinion leaders reinforce their commitment towards furthering cancer treatment standards. Cryoablation also aligns well with hospitals' focus on timely and effective care, lower readmission rates and improving overall patient experience. Strategic partnerships with community oncology centers can expand access to cryoablation therapies. A culture of providing world-class cancer care, enriching clinical expertise and ensuring best outcomes and experiences for patients drives a sizable portion of cryoablation procedures to be performed in hospitals versus other end users.
Need a Different Region or Segment? Download Free Sample
North America dominates the global cryoablation devices market with an estimated market share of 40.7% in 2025. The region accounts for the largest market share, owing to the highly developed healthcare infrastructure and higher adoption of technologically advanced cryoablation devices. The presence of leading cryoablation device manufacturers in the U.S. also drives the market growth. Moreover, favorable reimbursement policies and increasing demand for minimally invasive procedures for treating cancer and cardiac arrhythmias have boosted demand.
Asia Pacific region is the fastest growing market for cryoablation devices globally. Factors such as large population base suffering from cancer and increasing focus on developing a robust healthcare system can drive the market growth. Rising medical tourism owing to availability of low-cost treatments and devices can boost demand from international patients. Several new local manufacturers are entering the market by offering devices at competitive prices. This has increased the affordability and accessibility of cryoablation technologies.
Cryoablation Devices Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 466.1 Mn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 14.2% | 2032 Value Projection: | USD 1,181.4 Mn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
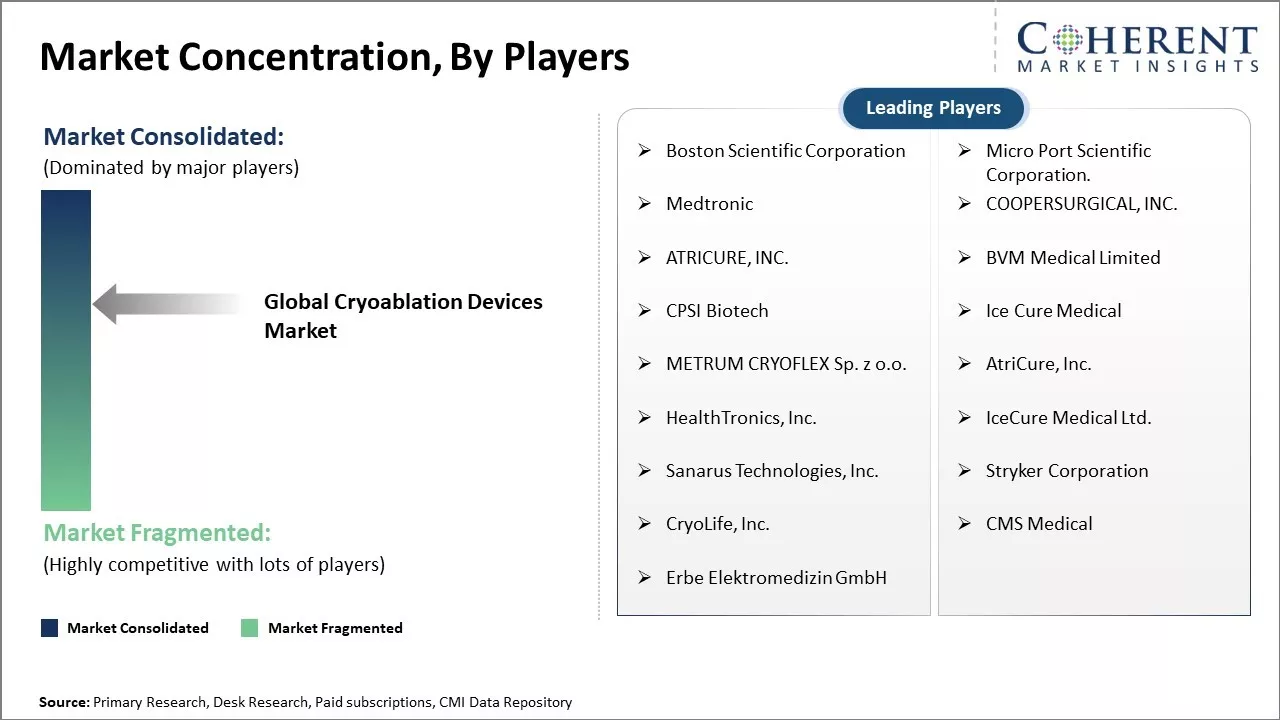
Boston Scientific Corporation, Micro Port Scientific Corporation., Medtronic, COOPERSURGICAL, INC., ATRICURE, INC., BVM Medical Limited, CPSI Biotech, Ice Cure Medical, METRUM CRYOFLEX Sp. z o.o., AtriCure, Inc., HealthTronics, Inc., IceCure Medical Ltd., Sanarus Technologies, Inc., Stryker Corporation, CryoLife, Inc., CMS Medical, Erbe Elektromedizin GmbH |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Share
Share
About Author
Komal Dighe is a Management Consultant with over 8 years of experience in market research and consulting. She excels in managing and delivering high-quality insights and solutions in Health-tech Consulting reports. Her expertise encompasses conducting both primary and secondary research, effectively addressing client requirements, and excelling in market estimation and forecast. Her comprehensive approach ensures that clients receive thorough and accurate analyses, enabling them to make informed decisions and capitalize on market opportunities.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients