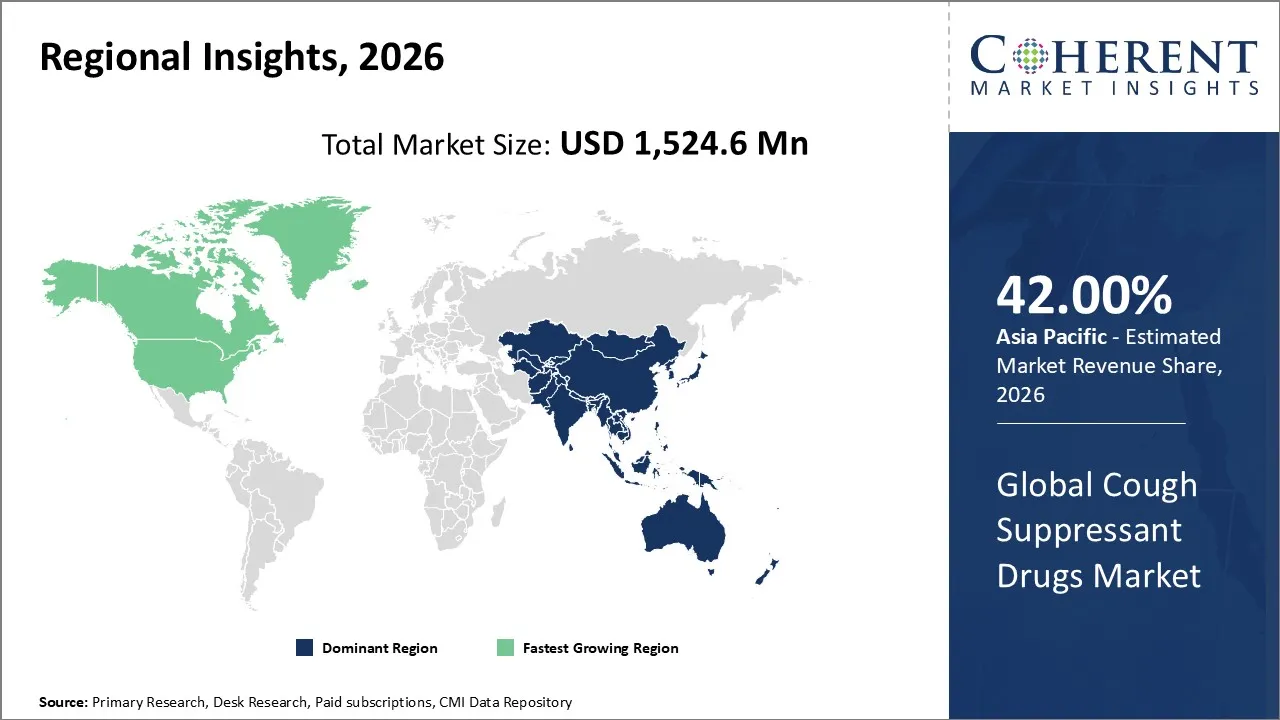
Cough suppressant drugs market is estimated to be valued at USD 1,524.6 Mn in 2026 and is expected to reach USD 1,966.1 Mn by 2033, exhibiting a compound annual growth rate (CAGR) of 3.7% from 2026 to 2033.
The rising prevalence of respiratory infections, seasonal flu, and allergies has increased demand for cough suppressant medications worldwide. The growing trend toward self-medication and easy availability of over-the-counter cough remedies have supplemented the market for cough remedies. Economies of scale can be achieved by the manufacturing companies through the launch of new formulations with quicker relief, improved taste, and better safety profile for adult and pediatric applications. Growing awareness about respiratory health, expanding retail and online pharmacy networks, and the rising geriatric population are expected to favorably impact the market.
|
Current Events |
and its impact |
|
AI-Driven Drug Discovery and Personalized Medicine Advancement |
|
|
Regulatory Harmonization and Safety Reassessments |
|
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Based on disease type, the dry cough segment is estimated to contribute the highest market share of 60% in 2026, owing to the higher prevalence of non-productive coughs caused by viral infections and allergies. Dry cough suppressants are still considered the best choice for both patients and physicians as they provide excellent relief in cases of annoying and persistent coughs and do not stimulate cough and mucus secretions.
For instance, in January 2026, Benadryl® launched a new variant, Benadryl® DR Dry Cough Active Relief, specifically formulated to target dry cough symptoms with a mentholated, sugar‑ and alcohol‑free formula, helping soothe irritated throats without causing drowsiness
In terms of drug type, the dextromethorphan segment is estimated to contribute the highest market share of 55% in 2026, as it is the most widely used active ingredient in cough suppressant formulations globally. Dextromethorphan medications come in various preparations like syrups, lozenges, and extended-release tablets, thus making it very versatile. This versatility is attributed to its widespread usage due to its efficacy, safety, and strong presence in over-the-counter medications.
For instance, in September 2024, ANI Pharmaceuticals, Inc. launched a new oral solution combining Promethazine Hydrochloride with Dextromethorphan Hydrobromide, strategically timed for the cough and cold season to offer broader symptomatic relief in a single formulation.
Based on product type, the over-the-counter (OTC) segment is estimated to contribute the highest market share of 60% in 2026, owing to strong self-medication trends and easier accessibility. OTC cough suppressants allow consumers to manage their symptoms in the comfort of their homes, thus offering convenience and driving volume growth. Pharmaceutical companies are continuously innovating with better taste, child-friendly formulations, and multi-symptom products to boost demand even more in this format.
For instance, in March 2025, Reckitt Benckiser announced a strategic partnership to co-market a new generation of OTC cough and cold products around the world, while Sanofi introduced a major new cough and cold OTC product in all the key European markets-a strategic move of both companies to further reinforce their OTC portfolio and distribution.
In terms of age group, the adult segment is estimated to contribute the highest market share of 50% in 2026, due to higher consumption of cough suppressants in the adult population. Adults comprise the largest consumer group for OTC and prescription cough medications, products that are influenced by lifestyle, work exposure to infections, and respiratory ailments that occur in certain months of the year. Formulations for such groups will typically include products for high dosages.
For instance, in September 2025, the Central Drugs Standard Control Organization (CDSCO) identified 112 drug samples that failed quality tests, including a spurious cough syrup product containing dextromethorphan and other ingredients.
In terms of distribution channel, the retail pharmacy segment is estimated to contribute the highest market share of 47% in 2026, owing to wide availability and frequent consumer purchasing. Retail pharmacies provide direct access to both OTC and prescription cough suppressants, supported by pharmacist recommendations and promotions. The convenience of nearby locations, coupled with in-store product variety, ensures retail pharmacies remain the preferred channel for cough suppressant purchases globally.
For instance, in December 2025, India's Union Health Ministry proposed a draft amendment aimed at excluding "syrups" (cough syrups) from the OTC drugs list under Schedule K of Drugs Rules. When finalized, cough syrups are to be sold only with a doctor's prescription and formal pharmacy licence, significantly altering how retail pharmacies can distribute cough suppressant syrups.
To learn more about this report, Download Free Sample
The Asia Pacific market is estimated to occupy the leading position in the cough suppressant market in 2026, accounting for an estimated market share of 42% in the market. The growth in the market is fueled by the high incidence of respiratory diseases, increasing healthcare expenditure, increasing awareness of self-medication practices, and the growth of the pharmaceutical retail industry.
For instance, in March 2025, Abbott launched a new range of herbal-based cough syrups in India to target adults and children. Faster relief and safety are the focus of the line, which melds traditional remedies with modern formulations and is distributed through retail pharmacies and e-commerce platforms throughout Asia Pacific.
North America is also expected to be the largest growing market for cough suppressant medications, and the reason includes a better healthcare infrastructure, a relatively high per capita spending on OTC medications, increased awareness about seasonal flu and respiratory conditions, and a widespread retail pharmacy infrastructure. Innovations in cough suppressant medications, such as combination syrups, sugar-free, and adult version medicines, also contribute to a growing market.
For instance, in July 2025, Pfizer introduced a new dextromethorphan-based adult cough syrup in the U.S. with enhanced delivery for faster relief. The product is available across retail pharmacies and online platforms, reflecting the shift toward convenient adult-oriented cough treatments.
The U.S. cough suppressant drugs market is experiencing steady growth, driven by factors such as a high prevalence of respiratory illnesses, widespread availability of over‑the‑counter (OTC) products, and robust healthcare spending that supports access to self‑care medications. Consumers increasingly turn to accessible cough remedies without prescriptions, supported by well‑established retail pharmacy networks and digital health platforms.
For instance, in December 2025, the U.S. Food and Drug Administration (FDA) approved a combination medication containing naproxen/dextromethorphan/guaifenesin under the brand name Mucinex 12HR Cold & Fever Multi‑Symptom, enhancing therapeutic options available to adults seeking multi‑symptom relief.
China remains a key driver of the Asia‑Pacific cough suppressant drugs market amid expanding urban healthcare access, rising disposable incomes, and increasing awareness of respiratory ailments. Rapid urbanization and a growing middle class are fueling demand for both OTC and prescription cough medicines, particularly in major cities with high air pollution and respiratory disease rates.
For instance, in February 2024, China’s National Medical Products Administration (NMPA) approved an innovative traditional Chinese medicine (TCM) cough syrup called Jiuwei Zhike Koufuye for marketing.
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2025 | Market Size in 2026: | USD 1,524.6 Mn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2026 To 2033 |
| Forecast Period 2026 to 2033 CAGR: | 3.7% | 2033 Value Projection: | USD 1,966.1 Mn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Perrigo Company plc, Vernalis plc, Tris Pharma Inc., Pfizer Inc., Aytu BioScience, Inc., Acella Pharmaceuticals LLC, Mayne Pharma Inc., Taro Pharmaceutical Industries Ltd., Amneal Pharmaceuticals LLC, Aurobindo Pharma Ltd, and GlaxoSmithKline plc. |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
The increasing cases of respiratory infections, allergies, and seasonal flu have catalyzed stiff demand for cough suppressant medications across the world. Apart from adults, pediatric cases have also shown increasing dependence on over-the-counter and prescription medications for relief, while public health consciousness to a large extent supports market growth.
The market presents opportunities for growth through the development of novel formulations, including herbal-based syrups, sugar-free liquids, fast-dissolving tablets, and combination medicines targeting multiple symptoms. Increasing options that are both appealing to adults and pediatrics, coupled with convenient packaging for store and online sales, can thus capture more significant shares of the market.
Share
Share
About Author
Vipul Patil is a dynamic management consultant with 6 years of dedicated experience in the pharmaceutical industry. Known for his analytical acumen and strategic insight, Vipul has successfully partnered with pharmaceutical companies to enhance operational efficiency, cross broader expansion, and navigate the complexities of distribution in markets with high revenue potential.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients