Constipation Treatment Market Size and Forecast – 2026 – 2033
The Global Constipation Treatment Market size is estimated to be valued at USD 5.2 billion in 2026 and is expected to reach USD 8.9 billion by 2033, exhibiting a compound annual growth rate (CAGR) of 7.3% from 2026 to 2033.
Global Constipation Treatment Market Overview
The global constipation treatment market is witnessing steady growth due to increasing prevalence of functional and chronic constipation across all age groups. Rising awareness about gastrointestinal health, lifestyle changes, and dietary factors are driving demand for effective treatments, including laxatives, prokinetic agents, and novel therapies. Hospitals and clinics remain primary treatment settings, while OTC products and digital health platforms are enhancing patient access. Innovation in drug formulations, including targeted and combination therapies, is improving efficacy and adherence. Growing healthcare expenditure, supportive reimbursement policies, and rising geriatric populations in developed and emerging regions are further fueling market expansion, making it a dynamic and evolving sector.
Key Takeaways
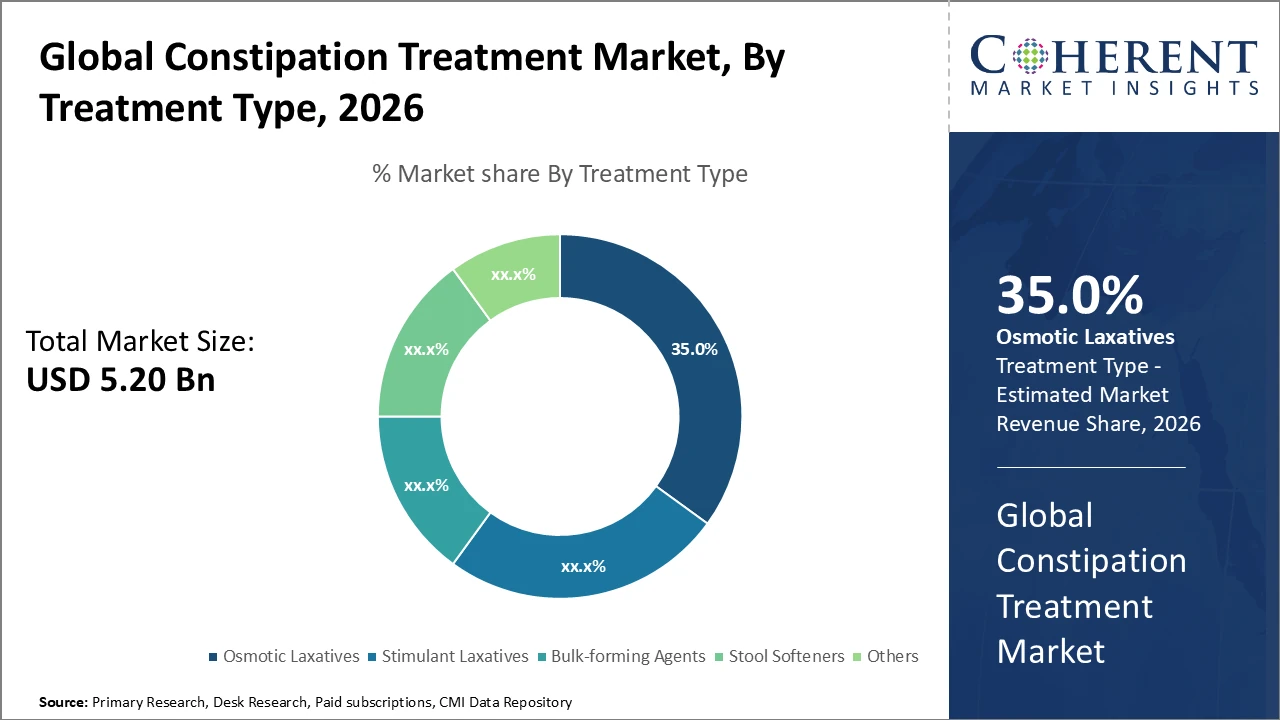
The osmotic laxatives segment dominates the market with a 35% share, driven by proven efficacy across all demographics.
Oral administration is the preferred route due to convenience and high patient compliance.
Hospitals and clinics are the largest end-user segment, contributing over 60% of market revenue, supported by institutional adoption of advanced treatment protocols.
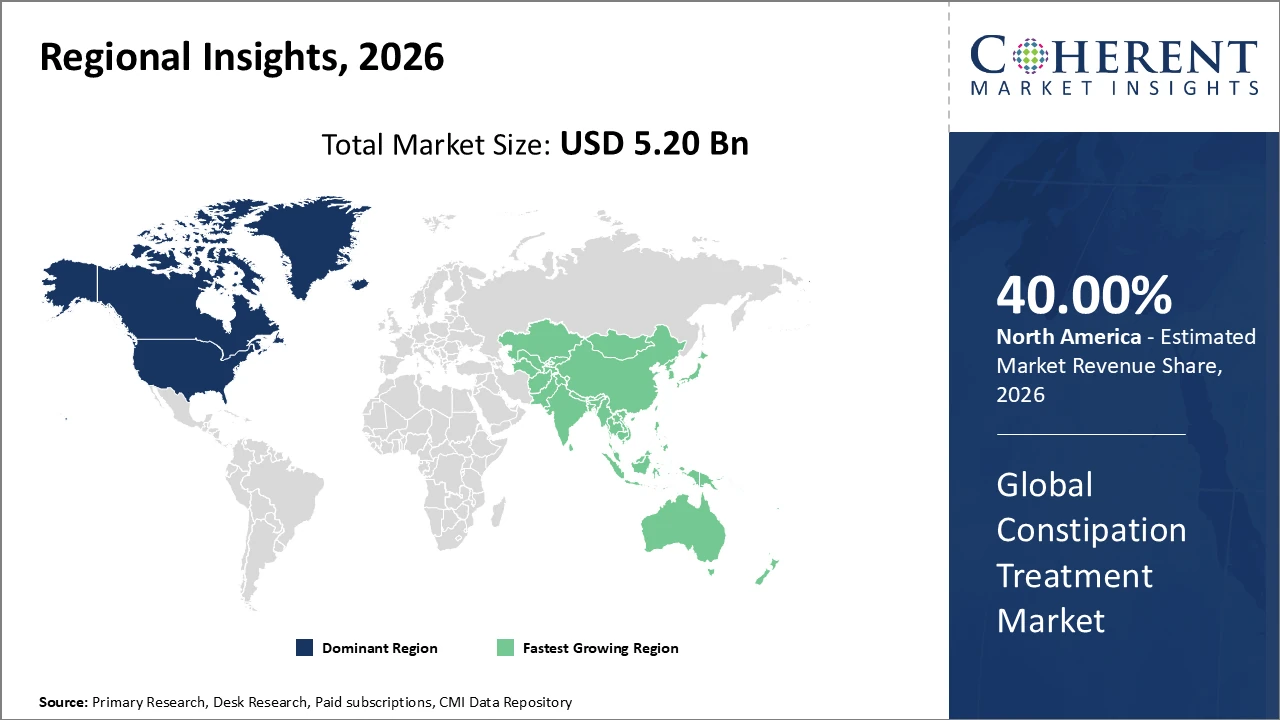
North America leads the market with over 40% share, backed by established healthcare infrastructure and regulatory frameworks enabling faster product approvals.
Asia Pacific is the fastest-growing region with a CAGR exceeding 9%, driven by rising healthcare expenditure, increasing patient awareness, and expanding pharmaceutical manufacturing.
Europe shows steady growth, supported by aging populations and government healthcare programs targeting gastrointestinal diseases.
Constipation Treatment Market Segmentation Analysis
To learn more about this report, Download Free Sample
Constipation Treatment Market Insights, By Treatment Type
Osmotic laxatives dominate the market with a 35% share, driven by their ability to draw water into the colon, facilitating easier stool passage, making them preferred for both chronic and acute constipation. The fastest-growing subsegment is stimulant laxatives, valued for their rapid action and increasing use in specific patient groups, such as those with opioid-induced constipation. Bulk-forming agents remain important for patients seeking gentle, fiber-based relief, while stool softeners are primarily used in postoperative and pediatric cases. The ‘Others’ category includes emerging herbal and enzyme-based formulations, reflecting rising interest in natural and alternative therapies.
Constipation Treatment Market Insights, By Route of Administration
Oral administration dominates the constipation treatment market due to patient preference for convenience, accessibility, and ease of use. Advances in formulations, such as sustained-release tablets and granules, have improved adherence to oral therapies. Rectal administration serves a supportive role, particularly in acute cases or for patients unable to take oral medications, and is steadily growing in clinical settings. Injectable forms are used in specialized cases requiring rapid onset but account for a minor share. Overall, improvements in oral dosage quality, efficacy, and minimized side effects continue to drive the growth of this segment.
Constipation Treatment Market Insights, By End-User
Hospitals dominate the constipation treatment market due to institutional access to advanced treatment formulations and a large patient population with chronic gastrointestinal issues. Clinics and outpatient facilities are gaining traction through the adoption of evidence-based protocols specifically for constipation management. The fastest-growing subsegment is home care settings, driven by telehealth expansion and increasing patient preference for home-based therapies, often supported by digital adherence monitoring tools. The ‘Others’ category includes specialized care centers focusing on geriatric and pediatric patients with tailored therapeutic approaches. These trends reflect a market adapting to both institutional demands and patient-centric care preferences.
Constipation Treatment Market Trends
Integration of digital therapeutics with traditional constipation treatments reflects a shift toward holistic, patient-centered care.
Wearable devices and smartphone apps for monitoring bowel habits have gained popularity, with adoption rising 25% among millennial patients between 2024 and 2026.
Personalized medicine approaches using microbiome analysis are emerging, with 2025 pilot studies showing a 30% improvement in patient outcomes.
Non-pharmacological options, including biofeedback therapy and dietary interventions, are gaining acceptance, particularly in developed regions.
Overall, these trends indicate a move toward precision healthcare and integrated patient management in constipation treatment.
Constipation Treatment Market Insights, By Geography
To learn more about this report, Download Free Sample
North America Constipation Treatment Market Analysis and Trends
North America dominates the constipation treatment market, accounting for over 40% of global share, driven by advanced healthcare infrastructure, high per capita spending, and rapid adoption of innovative therapies. The region benefits from the strong presence of leading companies such as Johnson & Johnson and Pfizer, which actively expand product portfolios and distribution networks. Government health initiatives focusing on aging populations and chronic gastrointestinal conditions further support market growth, ensuring widespread access to treatments and reinforcing North America’s leadership in the global constipation treatment market.
Asia Pacific Constipation Treatment Market Analysis and Trends
Asia Pacific is the fastest-growing region in the constipation treatment market, with a CAGR exceeding 9%. Growth is driven by expanding healthcare access, rising disposable incomes, and the development of pharmaceutical manufacturing hubs in countries such as China and India. Significant investments in healthcare infrastructure, coupled with increasing awareness of gastrointestinal health, are further accelerating market expansion. These factors position Asia Pacific as a key growth epicenter, attracting both local and international market players aiming to capitalize on the rising demand for effective constipation treatments.
Constipation Treatment Market Outlook for Key Countries
USA Constipation Treatment Market Analysis and Trends
The USA plays a major role in the global constipation treatment market, supported by healthcare expenditure exceeding USD 4 trillion annually. Key drivers include a large aging population susceptible to chronic constipation and a well-established pharmaceutical sector focused on innovative drug development. In 2025, prescription stimulant laxative usage rose by 15%, reflecting increased demand and treatment adoption. Leading companies such as Bayer and AbbVie maintain strong market presence, advancing innovation through pipeline products and strategic collaborations. Robust healthcare infrastructure, high patient awareness, and continuous product development collectively reinforce the USA’s leadership in the constipation treatment market.
Germany Constipation Treatment Market Analysis and Trends
Germany’s constipation treatment market is growing steadily, supported by a well‑established pharmaceutical industry and comprehensive healthcare coverage that enhances patient access to both prescription and OTC therapies. Functional constipation remains prevalent among the aging German population, contributing to sustained demand for laxatives and related treatments. Regulatory harmonization under the European Medicines Agency and local coverage through statutory health insurance ensure broad availability of therapeutic options, while increased research funding fosters innovation in personalized and advanced therapies. Consumer preference for natural and plant‑based digestive remedies and digital health solutions also supports market expansion and diversified treatment adoption.
Analyst Opinion
Rising demand for targeted laxative therapies is boosting market size, with prescription stimulant laxatives recording a 12% sales increase in 2025, driven largely by chronic idiopathic constipation cases.
Production capacity expansions by leading pharmaceutical companies in 2026 reflect a robust supply side; for example, a major North American plant increased output by 18% in 2025 to meet growing consumption in key markets.
Import volumes of novel prokinetic agents into emerging markets rose by 22% in 2024, highlighting a shift toward mechanism-based therapies and expanding market scope in Asia Pacific and Latin America.
Diverse therapeutic applications in elderly care and pediatric gastroenterology have broadened revenue streams, with pediatric formulations accounting for 15% of total sales growth in 2026, indicating evolving treatment use cases.
Market Scope
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2025 | Market Size in 2026: | USD 5.2 billion |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2026 To 2033 |
| Forecast Period 2026 to 2033 CAGR: | 7.3% | 2033 Value Projection: | USD 8.9 billion |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: | Johnson & Johnson, Pfizer, Bayer AG, AbbeVie Inc., AstraZeneca, Novartis International AG, Sun Pharmaceutical Industries Ltd., Mylan N.V., Shire plc, Allergan plc, Endo International plc | ||
| Growth Drivers: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Constipation Treatment Market Growth Factors
Market growth is largely driven by the rising prevalence of chronic constipation among the geriatric population, with global health data showing nearly a 20% increase in individuals over 65 affected by constipation-related disorders by 2026. Urbanization and sedentary lifestyles further contribute to higher incidence rates, especially in developed regions. Advances in drug delivery systems, such as sustained-release formulations, have enhanced therapeutic adherence, boosting market revenue. Additionally, growing awareness through digital health campaigns, increased screening by healthcare providers, and supportive government policies in emerging economies have expanded access to constipation treatments and accelerated overall market growth.
Constipation Treatment Market Development
In 2024, the constipation treatment market saw increased innovation in mechanism‑based therapies. Several pharmaceutical companies advanced clinical programs for next‑generation prokinetic agents that target underlying motility dysfunctions rather than solely providing symptomatic relief. Early phase trial results reported improved bowel movement frequency and patient‑reported outcomes, indicating potential shifts away from traditional laxative dominance toward more targeted pharmacological options.
Another key development in 2024 was the expansion of digital health solutions supporting constipation management. Telehealth services and mobile applications incorporating patient symptom tracking, personalized diet recommendations, and adherence reminders gained wider adoption among gastroenterologists and primary care physicians, enhancing patient engagement and improving long‑term treatment outcomes.
Key Players
Leading Companies of the Market
Johnson & Johnson
Bayer AG
Pfizer
AbbVie Inc.
AstraZenecaNovartis Internationa AG
Sun Pharmaceutical Industries Ltd.
Mylan N.V.
Shire Plc
Allergan plc
Several market players have pursued aggressive growth strategies through acquisitions and strategic partnerships. For example, in 2025, a leading pharmaceutical company acquired a biotech firm specializing in novel gut motility agents, driving a 14% revenue increase within one year. Additionally, collaborative R&D initiatives among global companies have accelerated the development and launch of next-generation constipation treatments with enhanced efficacy and safety profiles, strengthening their competitive positioning and market share across key regions. These strategies reflect a focus on innovation, portfolio expansion, and faster access to emerging therapeutic opportunities.
Constipation Treatment Market Future Outlook
The global constipation treatment market is expected to witness steady growth, driven by rising prevalence of chronic and functional constipation across all age groups. Advances in mechanism-based therapies, including prokinetic agents and novel stimulant formulations, are anticipated to expand treatment options. Digital health integration, such as telemedicine platforms and mobile applications for symptom tracking and adherence monitoring, will enhance patient engagement and personalized care. Growing awareness of gastrointestinal health, combined with supportive reimbursement policies and an aging population, will further fuel market expansion. Emerging markets in Asia Pacific and Latin America are projected to offer significant growth opportunities in the coming years.
Constipation Treatment Market Historical Analysis
The constipation treatment market has shown consistent growth over the past decade, driven by increasing incidence of both acute and chronic constipation globally. Early market development was dominated by traditional laxatives and bulk‑forming agents, with demand largely centered in developed regions due to greater healthcare access and diagnostic awareness. As understanding of gastrointestinal physiology improved, product innovation expanded to include prokinetic agents and targeted prescription therapies. Growing emphasis on patient quality of life and rising geriatric populations further accelerated adoption. Overall, historical trends reflect a transition from basic symptomatic treatments toward more diverse, mechanism‑based therapeutic options and integrated care approaches.
Sources
Primary Research Interviews:
Gastroenterologists
General Physicians
Pharmacists and Clinical Pharmacologists
Healthcare Consultants
Databases:
World Health Organization (WHO) Global Health Observatory
OECD Health Statistics
UN World Population Prospects
IMS Health / IQVIA Databases
Magazines:
Pharmaceutical Technology
PharmaTimes
Drug Discovery & Development
Pharmaceutical Executive
European Pharmaceutical Review
Journals:
American Journal of Gastroenterology
Neurogastroenterology & Motility
Gut
Alimentary Pharmacology & Therapeutics
Newspapers:
The New York Times (Health)
The Guardian (Health)
Financial Times (Healthcare)
The Hindu (Health)
Reuters Health
Associations:
World Gastroenterology Organisation (WGO)
European Society of Neurogastroenterology & Motility (ESNM)
Digestive Health Foundation
American Gastroenterological Association (AGA)
International Foundation for Gastrointestinal Disorders (IFFGD)
Share
Share
About Author
Abhijeet Kale is a results-driven management consultant with five years of specialized experience in the biotech and clinical diagnostics sectors. With a strong background in scientific research and business strategy, Abhijeet helps organizations identify potential revenue pockets, and in turn helping clients with market entry strategies. He assists clients in developing robust strategies for navigating FDA and EMA requirements.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients