Colon And Rectal Cancer Drugs Market Size and Forecast – 2025 – 2032
The Global Colon And Rectal Cancer Drugs Market size is estimated to be valued at USD 8.4 billion in 2025 and is expected to reach USD 14.6 billion by 2032, exhibiting a compound annual growth rate (CAGR) of 8.3% from 2025 to 2032.
Global Colon And Rectal Cancer Drugs Market Overview
Colon and rectal cancer drugs include a wide range of therapeutic formulations designed to inhibit tumor growth, prevent metastasis, and improve patient survival. These products encompass chemotherapeutic agents such as 5-fluorouracil and oxaliplatin, targeted therapies that block EGFR or VEGF signaling pathways, and next-generation immunotherapies that activate the body’s immune system against cancer cells. Oral and intravenous formulations provide dosing flexibility, while biosimilar introductions have made advanced therapies more affordable. The growing integration of genetic and biomarker-based testing supports personalized drug selection, improving treatment outcomes and minimizing side effects.
Key Takeaways
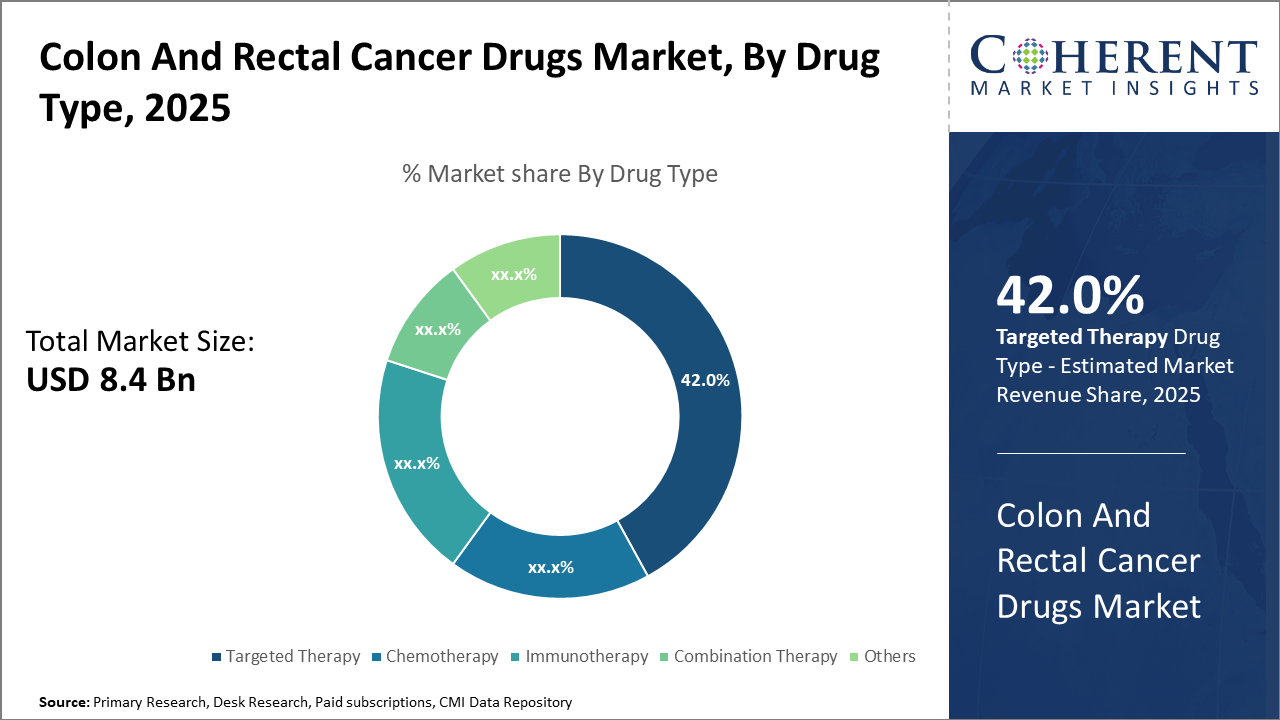
The targeted therapy segment dominates the colon and rectal cancer drugs market, accounting for 42% market share, propelled by the high specificity and improved efficacy of monoclonal antibodies and small molecules. Meanwhile, immunotherapy subsegments display the highest growth rate due to expanding clinical applications.
Intravenous administration remains the primary route in hospitals and clinics, but oral formulations are gaining momentum, reflecting patient preference for convenience and outpatient treatment models.
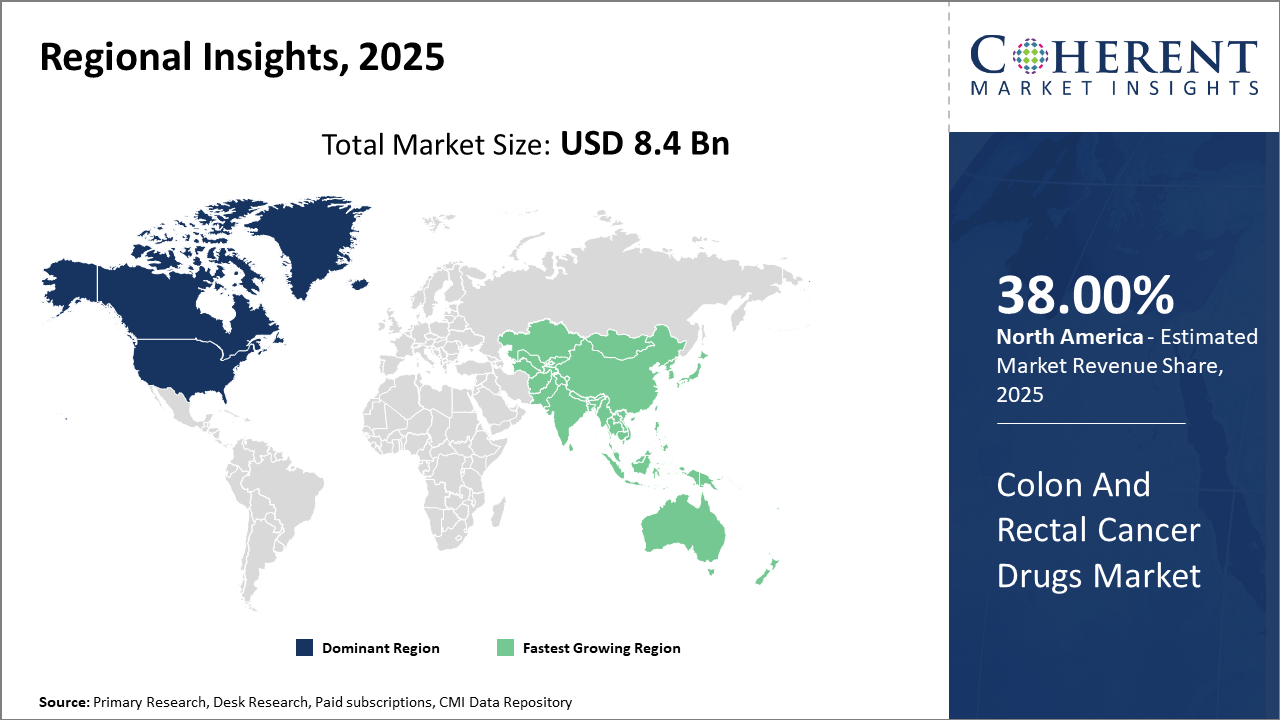
North America leads the regional market share with more than 38%, driven by significant investment in R&D, high healthcare expenditure, and well-established screening frameworks.
Asia Pacific emerges as the fastest-growing region, with a CAGR exceeding 9%, fueled by increasing disease burden, expanding healthcare infrastructure, and improving reimbursement policies in key countries like China and India.
Colon And Rectal Cancer Drugs Market Segmentation Analysis
To learn more about this report, Download Free Sample
Colon And Rectal Cancer Drugs Market Insights, By Drug Type
Targeted Therapy dominates the market share with 42%. This dominance is attributable to the precision and efficacy of monoclonal antibodies and small molecules designed for specific oncogenic pathways, offering superior progression-free survival rates. Immunotherapy, the fastest-growing subsegment, benefits from expanded indications such as immune checkpoint inhibitors, marking a paradigm shift from traditional drugs. Chemotherapy remains a foundational treatment due to its cost-effectiveness, but its use is declining slightly as patients and clinicians prefer targeted approaches.
Colon And Rectal Cancer Drugs Market Insights, By Route of Administration
Intravenous administration dominates the market due to the large volume of hospital and clinic-based infusions, ensuring controlled dosing and immediate medical supervision. However, Oral administration is the fastest-growing subsegment, driven by rising patient preference for at-home treatment and adherence benefits. Subcutaneous formulations are emerging, especially for monoclonal antibodies, offering convenient alternatives with comparable efficacy.
Colon And Rectal Cancer Drugs Market Insights, By End-User
Hospitals dominate the market share, benefiting from comprehensive oncology departments and infrastructure to administer complex intravenous therapies. Specialty oncology clinics are expanding rapidly, offering focused cancer care and personalized treatment protocols, and represent the fastest-growing subsegment by capitalizing on outpatient care trends and patient-centric models. Ambulatory surgical centers are gaining prominence for minor procedures and oral drug dispensing, reflecting a shift towards decentralization of cancer care.
Colon And Rectal Cancer Drugs Market Trends
Recent years have seen a notable acceleration in the adoption of immunotherapy in the colon and rectal cancer drugs market, with checkpoint inhibitors gaining approval for MSI-high colorectal cancer cases.
In 2024, regulatory approvals expanded to include first-line combination treatments, significantly increasing patient pool access.
Additionally, the integration of AI-powered platforms in drug discovery and clinical trial optimization has streamlined the development process, reducing the average time to market by nearly 25%.
Another trend is the increasing use of oral targeted therapies, catering to patient convenience and improving adherence, evidenced by a 12% rise in oral drug prescriptions in 2024.
Colon And Rectal Cancer Drugs Market Insights, By Geography
To learn more about this report, Download Free Sample
North America Colon And Rectal Cancer Drugs Market Analysis and Trends
In North America, the dominance in the Colon And Rectal Cancer Drugs Market is reinforced by strong government funding for cancer research and high healthcare spending. The U.S. alone accounts for approximately 30% of global market revenue, supported by advanced screening programs and early-stage diagnosis infrastructure. The presence of leading biotech and pharma companies ensures continual pipeline innovation and market leadership.
Asia Pacific Colon And Rectal Cancer Drugs Market Analysis and Trends
Meanwhile, the Asia Pacific exhibits the fastest growth with a CAGR above 9%, driven mainly by rising incidence rates, improving healthcare access, and growing pharmaceutical manufacturing capabilities. Countries like China and India are witnessing enhanced regulatory frameworks and increased awareness campaigns, accelerating drug uptake.
Colon And Rectal Cancer Drugs Market Outlook for Key Countries
USA Colon And Rectal Cancer Drugs Market Analysis and Trends
The USA’s market remains the largest contributor globally, reflecting strong oncology drug adoption and advanced healthcare infrastructure. In 2024, the FDA approved multiple new drugs targeting specific genetic mutations in colorectal cancer, contributing to a 7% increase in annual market revenue. Leading companies such as Amgen and Merck dominate with innovative launches focusing on immunotherapy and targeted agents. The presence of comprehensive insurance coverage further facilitates patient access, underpinning sustained business growth.
Germany Colon And Rectal Cancer Drugs Market Analysis and Trends
Germany’s market features a well-established healthcare system and proactive screening programs, positioning it as a key regional contributor in Europe. In 2024, reimbursement reforms enhanced the accessibility of novel colon and rectal cancer drugs, resulting in a 6% market uplift. Biotech collaborations, particularly around personalized medicine, have expedited market introduction of next-generation therapies. The country also benefits from its central location, acting as a logistical hub for pharmaceutical distribution across Europe.
Analyst Opinion
The rising incidence rate of colorectal cancer, particularly in developed countries, continues to be a principal demand-side driver impacting the market share and revenue. As per recent 2024 data from the American Cancer Society, colorectal cancer ranks as the third leading cause of cancer-related deaths, signaling sustained demand for innovative drug therapies. Additionally, the expansion of screening programs is enhancing early diagnosis, thereby broadening therapeutic intervention opportunities. For instance, the U.S. saw a 5% increase in colorectal cancer detection in early stages in 2024, fueling growth in drug consumption.
On the supply-side, advancements in biologic therapies and immuno-oncology treatments are significantly expanding pipeline capacities. In 2025, several monoclonal antibodies and immune checkpoint inhibitors designed specifically for colon and rectal cancers are projected to launch, increasing production capacity by an estimated 12%. This supply expansion further boosts market revenue by providing diversified therapeutic options.
The integration of companion diagnostics to tailor treatment plans based on genetic biomarkers is transforming market dynamics. For example, precision oncology adoption rates surged by over 18% in 2024 across Europe and North America, directly contributing to increased market size by enabling higher efficacy treatments and patient adherence. This demand for biomarker-driven therapies is likely to alter market share allocation among drug classes.
Import-export trends are balancing regional supply-demand asymmetries. In Asia Pacific, pharmaceutical imports of targeted colon and rectal cancer drugs grew by 15% in 2024, reflecting a growing patient base and insufficient domestic production. Conversely, North America remains a net exporter of advanced oncology medications, underscoring its leadership in drug innovation and manufacturing.
Market Scope
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2025 | Market Size in 2025: | USD 8.4 billion |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 8.3% | 2032 Value Projection: | USD 14.6 billion |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: | Amgen Inc., Bayer AG, Pfizer Inc., Merck & Co., Inc., Roche Holding AG, Johnson & Johnson, Novartis AG, Bristol-Myers Squibb, AstraZeneca PLC, Sanofi S.A., Eli Lilly and Company. | ||
| Growth Drivers: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Colon And Rectal Cancer Drugs Market Growth Factors
Increasing colorectal cancer prevalence aligned with aging populations and lifestyle changes is a primary growth driver globally, with incidences rising by approximately 7% annually in many countries throughout 2024. Breakthroughs in targeted therapy and immunotherapy, supported by robust clinical trials, continue to enhance treatment efficacy, thereby driving drug adoption and market revenue growth. For example, the introduction of novel PD-1 inhibitors showed a 20% improvement in patient response rates in 2024 studies.
Expansion of healthcare infrastructure and reimbursement policies, especially in emerging economies like China and Brazil, is improving patient access to sophisticated colon and rectal cancer drugs, expanding the overall market size. Growing public and private investments in oncology R&D further expedite drug innovation cycles, fueling pipeline strength and enabling faster regulatory approvals for next-generation therapies.
Colon And Rectal Cancer Drugs Market Development
In December 2024, Encorafenib (Braftovi) in combination with cetuximab and mFOLFOX6 received accelerated approval from the U.S. FDA for the treatment of patients with metastatic colorectal cancer (mCRC) harboring the BRAF V600E mutation, as confirmed by an FDA-approved diagnostic test. This triplet regimen offers a targeted therapy option aimed at improving response rates and outcomes in patients with limited treatment alternatives.
In June 2024, Adagrasib (Krazati) in combination with cetuximab received accelerated FDA approval for adults with KRAS G12C-mutated locally advanced or metastatic colorectal cancer who have previously undergone fluoropyrimidine-, oxaliplatin-, and irinotecan-based chemotherapy. This combination provides a precision-medicine approach for a difficult-to-treat mutation, addressing an unmet clinical need in previously treated colorectal cancer patients.
Key Players
Leading Companies of the Market
Amgen Inc.
Bayer AG
Pfizer Inc.
Merck & Co., Inc.
Roche Holding AG
Johnson & Johnson
Novartis AG
Bristol-Myers Squibb
AstraZeneca PLC
Sanofi S.A.
Notably, companies like Amgen and Roche have adopted aggressive expansion strategies, such as strategic acquisitions and pipeline diversification, resulting in a 10% uptick in their market revenue share between 2023 and 2024. Moreover, Pfizer’s collaboration with biotechnology firms to fast-track immunotherapy trials has accelerated development timelines, reinforcing its competitive positioning within the colon and rectal cancer drugs landscape.
Colon And Rectal Cancer Drugs Market Future Outlook
The future of colon and rectal cancer drug development is expected to be defined by personalized medicine, combination regimens, and immunotherapy advancements. Next-generation sequencing will enable patient-specific treatment optimization, while emerging bispecific antibodies and cell-based therapies promise enhanced tumor selectivity. Increasing investment in adjuvant and neoadjuvant studies, along with rising global screening rates, will expand treatment demand. Biosimilars will improve accessibility, and digital treatment monitoring will support precision dosing and adherence. Collectively, these advances are anticipated to improve long-term survival while transforming cancer care into a more individualized, data-driven discipline.
Colon And Rectal Cancer Drugs Market Historical Analysis
Historically, colon and rectal cancer therapies were dominated by traditional chemotherapeutic regimens such as 5-fluorouracil (5-FU) combined with leucovorin and oxaliplatin or irinotecan. These agents, while effective, offered limited specificity and often caused significant systemic toxicity. The early 2000s marked a pivotal era with the introduction of monoclonal antibodies targeting VEGF (bevacizumab) and EGFR (cetuximab), which significantly improved overall survival in advanced disease stages. Over time, the understanding of tumor genomics drove the emergence of precision oncology, enabling the use of biomarker-guided therapy. The expansion of clinical research, along with improved diagnostic tools, reshaped treatment paradigms from broad-spectrum cytotoxic agents toward targeted molecular drugs and immune checkpoint inhibitors.
Sources
Primary Research Interviews:
Oncologists
Clinical Pharmacologists
Cancer Research Scientists
Pharmaceutical Executives
Databases:
NCI Cancer Statistics
GlobalData Oncology Pipeline
WHO Cancer Database
Magazines:
Cancer Therapy Advisor
Pharmaceutical Technology
Oncology Times
BioPharma Dive
Journals:
The Lancet Oncology
Journal of Clinical Oncology
Cancer Research
Nature Reviews Cancer
Newspapers:
The New York Times (Health)
The Guardian (Science)
The Hindu (Health)
The Wall Street Journal (Pharma)
Associations:
American Cancer Society (ACS)
World Health Organization (WHO)
American Society of Clinical Oncology (ASCO)
European Society for Medical Oncology (ESMO)
Share
Share
About Author
Vipul Patil is a dynamic management consultant with 6 years of dedicated experience in the pharmaceutical industry. Known for his analytical acumen and strategic insight, Vipul has successfully partnered with pharmaceutical companies to enhance operational efficiency, cross broader expansion, and navigate the complexities of distribution in markets with high revenue potential.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients