Wilson’s disease is a rare genetic disorder characterized by excess copper stored in various body tissues, particularly in the liver, brain, and corneas of the eyes. The disease is progressive, and if left untreated, it may cause liver (hepatic) disease, central nervous system dysfunction, and death. The signs and symptoms of Wilson’s disease usually first appear between the ages of 6 and 45 years; however, they most often begin during the teenage years. Wilson’s disease is also known as hepatolenticular degeneration and lenticular degeneration. Early diagnosis and treatment prevent serious long-term disability and life-threatening complications. According to the National Organization for Rare Disorders, Inc. (NORD), Wilson’s disease is a rare disorder that affects males and females in equal numbers. The disease is found in all races and ethnic groups. The estimates vary; however, it is believed that Wilson’s disease occurs in approximately one in 30,000 to 40,000 people worldwide, according to NORD.
Colombia, Chile and Peru Wilson’s Disease Treatment Market - Impact of the Coronavirus (COVID-19) Pandemic
The COVID-19 pandemic has negatively impacted the growth of Colombia, Chile and Peru Wilson’s disease treatment market. Treatment for Wilson’s disease should be taken daily and continued lifelong. A patient needs to visit hospitals for the clinical examination, ultrasound of the liver, and urine samples every six months in the maintenance phase of the disease. The patient also needs to visit more frequently to the hospital when the treatment is just initiated. Due to the COVID-19 pandemic, government of respective countries imposed the lockdown on the entire nations respectively. This lockdown affected the treatments of many patients with diseases, including patients with Wilson's disease. The non-urgent care of these patients was therefore suspended. Medical consultations and paramedical care (physiotherapy, speech therapy, psychologist, etc.) have been postponed. Only urgent hospitalizations in the event of imbalance of patients’ illness with life-threatening risk were maintained. In this situation, Wilson's disease patients could be particularly anxious.
Moreover, due to the lockdown, the recruitment for clinical trials for overall diseases was halted. Thus, the COVID-19 has negatively impacted Colombia, Chile and Peru Wilson’s disease treatment market.
The increasing number of research and development activities to treat Wilson’s disease is expected to drive the growth of Colombia, Chile and Peru Wilson’s disease treatment market over the forecast period. For instance, in September 2017, according to The Wilson Disease Association, patients with Wilson’s disease were being recruited by Wilson Disease Association, for a Pilot study to evaluate brain changes using magnetic resonance imaging (MRI). The study was sponsored by The Wilson Disease Association.
Colombia, Chile and Peru Wilson’s disease treatment market is estimated to be valued at US$ 799.4 thousand in 2022 and expected to exhibit a CAGR of 5.1% over the forecast period (2022-2030).
Figure 1: Colombia, Chile and Peru Wilson’s Disease Treatment Market Share (%) Analysis, By Drugs, 2021
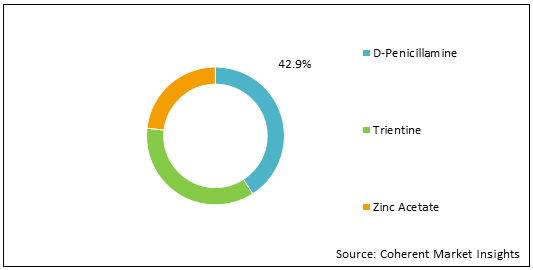
To learn more about this report, Download Free Sample
The increasing number of generic drug launches by the market players is expected to drive the growth of Colombia, Chile and Peru Wilson’s disease treatment market over the forecast period.
Market players are focused on organic growth strategies such as drug launches, which is expected to drive the market growth over the forecast period. For instance, in February 2018, Teva Pharmaceutical Industries Ltd., a pharmaceutical company, announced the launch of generic version of Syprine (trientine hydrochloride) capsules, 250 mg, in the U.S. Trientine hydrochloride is used in the treatment of patients with Wilson’s disease who are intolerant of penicillamine.
Colombia, Chile and Peru Wilson’s Disease Treatment Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2021 | Market Size in 2022: |
US$ 799.4 K |
| Historical Data for: | 2017 to 2020 | Forecast Period: | 2022 to 2030 |
| Forecast Period 2022 to 2030 CAGR: | 5.1% | 2030 Value Projection: |
US$ 1,190.2 K |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Teva Pharmaceuticals Industries Ltd., Bausch Health Companies Inc., Alexion Pharmaceutical, Inc., and Sanofi S.A. |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
The increasing number of drug approvals from regulatory bodies is expected to drive the growth of Colombia, Chile and Peru Wilson’s disease treatment market over the forecast period.
Increasing approvals from regulatory authorities are expected to drive the growth of Colombia, Chile and Peru Wilson’s disease treatment market over the forecast period. For instance, in May 2019, Amerigen Pharmaceuticals Limited., a generic pharmaceutical company, announced that Amerigen's Abbreviated New Drug Application (ANDA) for Penicillamine Capsules USP 250 mg has received final approval from the U.S. Food and Drug Administration (FDA). This is the first such ANDA to be approved as a generic equivalent to Bausch Heath's Cuprimine.
Colombia, Chile and Peru Wilson’s Disease Treatment Market – Restraints
The high price of drugs (including branded and generic) for the treatment of Wilson’s disease and low affordability among patients for such drugs in Colombia, Chile, and Peru is a major hurdle for the market growth. For instance, in 2018, Teva Pharmaceutical Industries Ltd., a pharmaceutical company, launched the generic version of Syprine (branded drug for Wilson disease) in the U.S., which costs US$ 18,275 for 100 pills, a cheaper alternative to treat Wilson’s disease by less than US$ 3,000. However, this price is too high for patients in countries such as Chile, Colombia, and Peru. Thus, the launch of low price generics can increase the affordability for treatment among the population in these countries.
The lack of proper regulatory policies is also one of the major restraining factors for the market growth in Colombia, Chile, and Peru. For instance, in 2016, Latin American countries collaborated with the Pan American Health Organization (PAHO), which recognized Colombia to have national regulatory agencies that are competent and performant. However, despite this recognition of progress, the duration required to obtain product approval is relatively slow in the country. Therefore, no approvals of drugs from regulatory bodies abstain patients from treatments of rare diseases such as Wilson’s disease.
Colombia, Chile and Peru Wilson’s Disease Treatment Market – Country Analysis
On the basis of country, Colombia, Chile and Peru Wilson’s disease treatment market is segmented into Colombia, Chile, and Peru.
Colombia is expected to hold a dominant position in the market during the forecast period, owing to increasing expenditure on healthcare by the government. For instance, according to the report of World Data Atlas, in 2018, health expenditure per capita for Colombia was US$ 513. Health expenditure per capita of Colombia increased from US$ 158 in 2004 to US$ 513 in 2018, growing at an average annual rate of 9.62%.
Figure 2: Colombia, Chile and Peru Wilson’s Disease Treatment Market (US$ Thousand), by Country, 2021
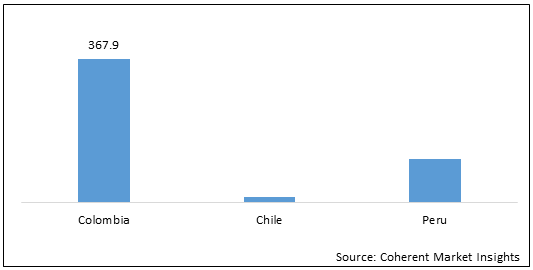
To learn more about this report, Download Free Sample
Colombia, Chile and Peru Wilson’s Disease Treatment Market – Competitive Landscape
Major players operating in Colombia, Chile and Peru Wilson’s disease treatment market include Teva Pharmaceutical Industries Ltd., Bausch Health Companies Inc., Alexion Pharmaceutical, Inc., and Sanofi S.A.
Share
Share
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients