Cold Plasma Bio Welding Device Market Size and Forecast – 2026 – 2033
The Global Cold Plasma Bio Welding Device Market size is estimated to be valued at USD 420 million in 2026 and is expected to reach USD 936 million by 2033, exhibiting a compound annual growth rate (CAGR) of 11.5% from 2026 to 2033.
Global Cold Plasma Bio Welding Device Market Overview
Cold plasma bio-welding devices are advanced surgical tools that use low-temperature plasma to join biological tissues without traditional sutures or staples. These devices create controlled plasma energy that facilitates tissue bonding while minimizing thermal damage. Products are used in minimally invasive surgeries, wound closure, and tissue repair applications. They offer advantages such as reduced bleeding, faster healing, and improved cosmetic outcomes compared to conventional surgical closure methods.
Key Takeaways
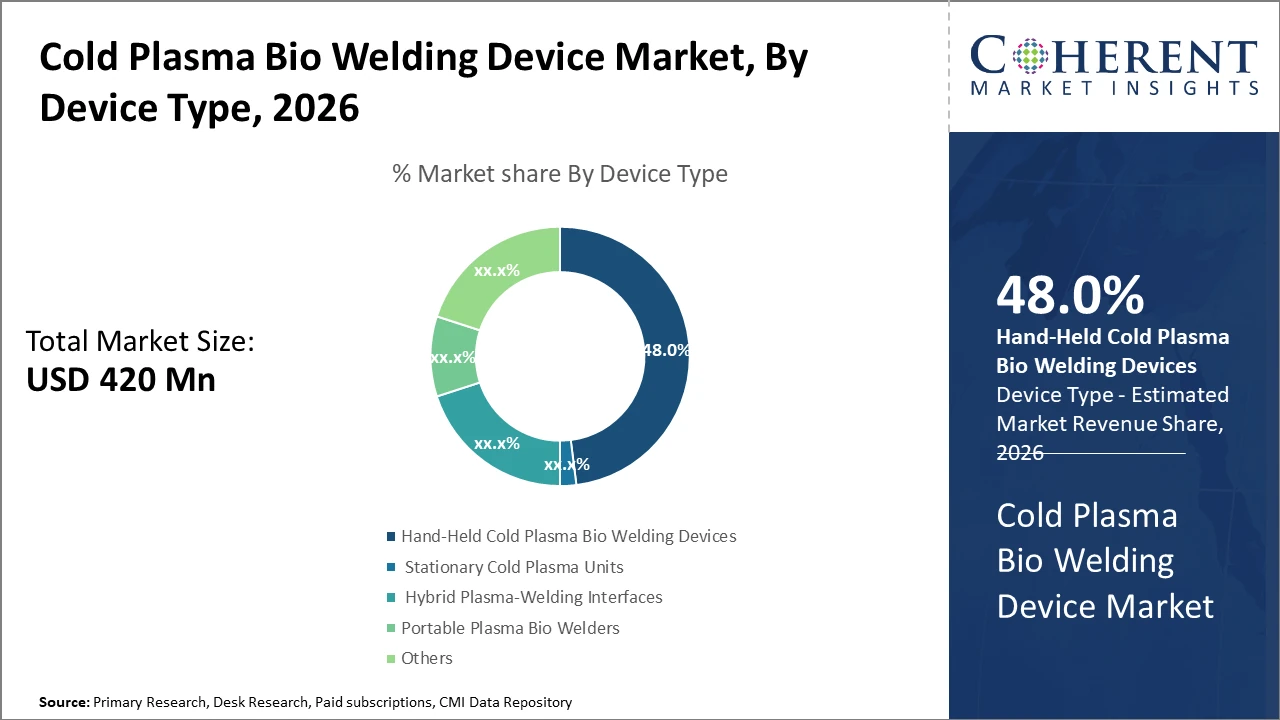
The hand-held cold plasma bio-welding device segment dominates due to its versatility and ease of use in diverse clinical settings, accounting for nearly half of the industry share.
Santizing surgical wound closure represents the largest application segment, driven by rising surgical interventions worldwide.
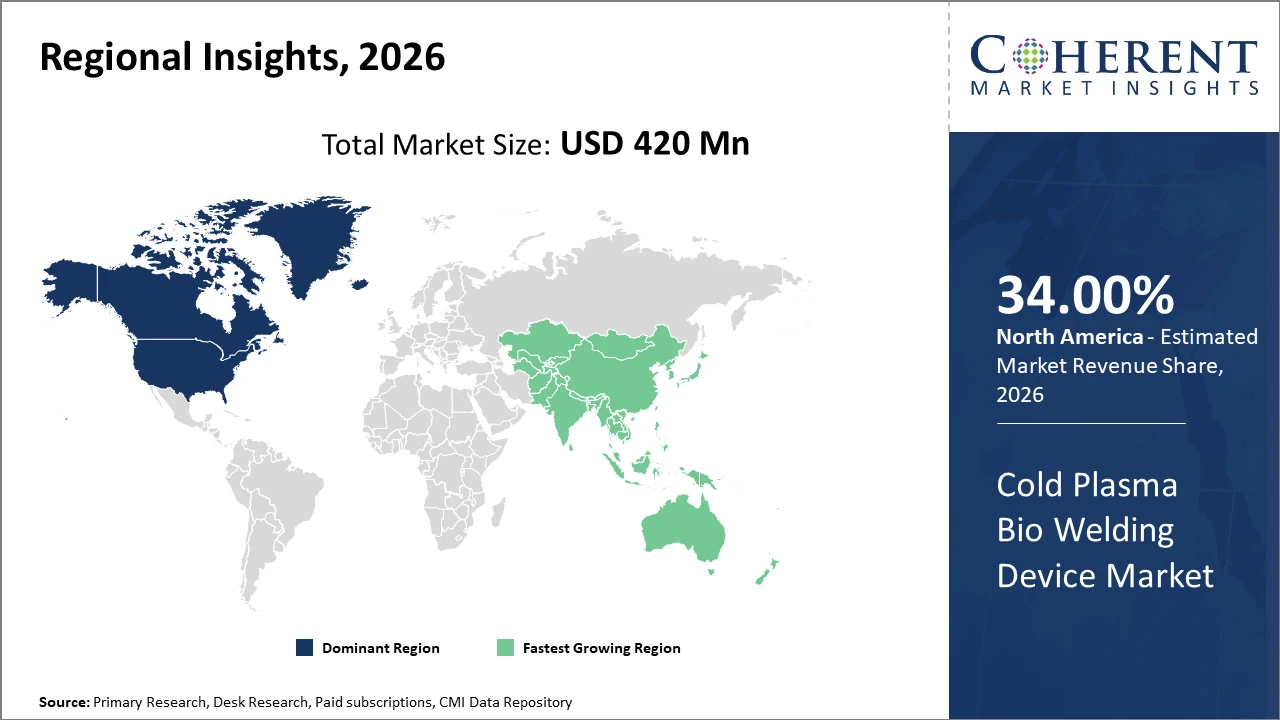
North America holds the largest regional market share by 34% given its advanced healthcare infrastructure and high adoption rates, while Asia Pacific is the fastest-growing region, propelled by escalating investments in healthcare infrastructure and rising chronic disease incidence.
Cold Plasma Bio Welding Device Market Segmentation Analysis
To learn more about this report, Download Free Sample
Cold Plasma Bio Welding Device Market Insights, By Device Type
Hand-held cold plasma bio-welding devices dominate the market share with approximately 48%. This dominance is driven by their operational flexibility, ease of use in diverse clinical settings, and suitability for minimally invasive procedures. Their compact design allows surgeons to have close operative control, enhancing precision in wound closure. The fastest growing subsegment is hybrid plasma-welding interfaces, which combine stationary plasma generators with mobile control units, offering enhanced performance for specialized surgeries owing to their adaptability and integration with surgical robotics.
Cold Plasma Bio Welding Device Market Insights, By Application
Surgical wound closure stands as the dominating application segment, accounting for the largest industry share, given the surge in minimally invasive surgeries worldwide. These devices enable sutureless closures, reducing surgical time and postoperative complications—key drivers for hospital adoption. The fastest growing application is dermatological treatments, particularly in cosmetic and chronic wound care segments, propelled by stronger patient demand and advancements in plasma technology targeting skin regeneration. Orthopedic repairs using cold plasma bio-welding are garnering interest but are still emerging due to complexity and device calibration needs.
Cold Plasma Bio Welding Device Market Insights, By End-User
Hospitals and clinics dominate this segment, accounting for the majority share, due to high procedure volumes and availability of specialized personnel trained to operate bio welding devices. Their focus on reducing postoperative complications and hospital stay length fuels sustained demand. Ambulatory surgical centers are the fastest-growing subsegment, benefitting from shifts towards outpatient care models and cost-efficient wound management solutions, leading to increased adoption of portable and hand-held plasma devices. Research and academic institutes mainly drive innovation and clinical validation, but contribute less to direct device revenue.
Cold Plasma Bio Welding Device Market Trends
The Cold Plasma Bio Welding Device market is experiencing significant shifts due to technological innovation and expanding healthcare access.
The adoption of AI-integrated plasma devices has elevated precision in surgical applications, with clinical trials in the U.S. showcasing a 10% reduction in operative errors by 2026.
Nano-plasma technology development represents another trend, enhancing microvascular repair, as demonstrated by devices launched in Japan and South Korea in 2024.
The regulatory environment is also evolving, with streamlined approval pathways in Asia Pacific encouraging device launches, accelerating market revenue growth by nearly 16% in that region.
Cold Plasma Bio Welding Device Market Insights, By Geography
To learn more about this report, Download Free Sample
North America Cold Plasma Bio Welding Device Market Analysis and Trends
In North America, the dominance in the Cold Plasma Bio Welding Device market is attributed to robust hospital infrastructure, high healthcare expenditure, and early adoption of innovative medical devices. Key players actively collaborate with academic institutions to develop highly specialized devices. Government initiatives promoting minimally invasive surgeries further fuel market expansion.
Asia Pacific Cold Plasma Bio Welding Device Market Analysis and Trends
Meanwhile, Asia Pacific exhibits the fastest growth with a CAGR of 14%, driven by rising healthcare infrastructure investments, increasing chronic disease prevalence, and evolving reimbursement frameworks in countries like India and China. Market companies have expanded regional distribution networks and localized manufacturing to capitalize on cost-effectiveness and scale.
Cold Plasma Bio Welding Device Market Outlook for Key Countries
USA Cold Plasma Bio Welding Device Market Analysis and Trends
The USA’s market leads in technological innovation and application diversity within the Cold Plasma Bio Welding Device domain. Market leaders such as PlasmaTech Medical Solutions and MedPlasma Systems have spearheaded product development focused on hybrid and hand-held devices, capturing significant market revenue. The U.S. healthcare ecosystem embraces rapid clinical validation, supporting the fastest adoption curve worldwide. Clinical collaborations led to over 30 institutional trial completions by 2025, emphasizing infection control and operational efficiency gains. The country’s favorable reimbursement policies and extensive outpatient surgical centers further underpin business growth.
Germany Cold Plasma Bio Welding Device Market Analysis and Trends
Germany’s Cold Plasma Bio Welding Device market is growing steadily, owing to its advanced healthcare system and strong medical device manufacturing base. Companies like BioWeld Innovations and NeoWeld Technologies have established R&D centers here, enhancing product customization for European standards. Government-centered initiatives promoting minimally invasive surgeries have led to a 20% increase in plasma bio welding utilization in 2025. The country’s strategic location supports access to the broader European market, bolstering export opportunities and supplying high-quality devices to neighboring countries.
Analyst Opinion
The escalation in hospital-acquired infections has accelerated demand for cold plasma bio welding devices, as these devices provide sterile, sutureless wound closure solutions. Recent clinical studies from 2025 highlight a 25% reduction in postoperative infection rates when using plasma welding compared to traditional suturing methods.
The rising adoption of minimally invasive surgeries has fueled technology integration within healthcare facilities, with cold plasma bio welding devices being preferred for delicate procedures. For instance, the use of plasma welding in ophthalmic surgeries surged by 18% in 2026, as reported by leading surgical centers.
Supply chain advancements involving specialized bio-compatible electrodes and plasma generators have increased production capacity by 30% in 2024, lowering device costs by nearly 12%. This improvement has consequently expanded market penetration in mid-sized hospitals globally.
Demand-side factors such as growing geriatric populations in developed economies, have contributed to increased device utilization for chronic wound management, with market revenue witnessing a 15% rise in North America in 2025, supported by demographic trends.
Market Scope
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2025 | Market Size in 2026: | USD 420 million |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2026 To 2033 |
| Forecast Period 2026 to 2033 CAGR: | 11.5% | 2033 Value Projection: | USD 936 million |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: | BioFusion Technologies, NovoWeld Solutions, MedWeld Technologies, PlasmaHeal Systems, WoundTech Plasma Devices, BioPlasma Medical, NextGen Cold Plasma Devices, SurgiHeal Technologies, PlasmaBond Medical Systems, BioPlasma Innovations Inc. | ||
| Growth Drivers: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Cold Plasma Bio Welding Device Market Growth Factors
The demand for sutureless surgical techniques is a primary growth driver, as cold plasma bio-welding devices reduce operation time and postoperative complications—clinics adopting this technology reported a 20% improvement in procedural workflow efficiency in 2026. Secondly, increasing research funding into plasma medicine has accelerated innovations, with several clinical trials conducted in 2024 highlighting the efficacy of plasma welding in tissue regeneration, especially in chronic diabetic wounds. Thirdly, the expanding penetration of outpatient surgical centers in the Asia Pacific is driving demand, with a noted CAGR of 14% in the region due to favorable reimbursement policies and growing healthcare infrastructure. Finally, the rising prevalence of chronic injuries and growing geriatric patient base worldwide increases reliance on advanced wound management solutions, making cold plasma bio-welding devices pivotal to market growth.
Cold Plasma Bio Welding Device Market Development
In May 2024, US Medical Innovations, LLC received FDA 510(k) clearance for the Canady Helios Cold Plasma™ (CHCP) Ablation System, a surgical platform designed for the ablation of soft tissue. The system leverages cold plasma technology to enable precise tissue removal while minimizing thermal damage, supporting safer surgical interventions and improved procedural control across a range of operative settings.
In June 2025, Hyped Science GmbH launched the PHLAS Medical Aesthetic Device, a handheld solution powered by patented SMD cold plasma technology for skin rejuvenation in home care environments. The device targets growing consumer demand for non-invasive, clinically inspired aesthetic treatments, offering controlled plasma-based skin stimulation to improve skin appearance and texture outside traditional clinical settings.
In April 2024, Mirari Doctor PTE LTD introduced the MIRARI® Cold Plasma System, a portable cold plasma device designed for skin rejuvenation and wound healing applications. The system’s recent approvals from the Thai FDA and Vietnam’s Ministry of Health underscore its regulatory progress in Southeast Asia and position it for broader adoption in dermatology and wound care across clinical and outpatient settings.
Key Players
Leading Companies of the Market
BioFusion Technologies
NovoWeld Solutions
MedWeld Technologies
PlasmaHeal Systems
WoundTech Plasma Devices
BioPlasma Medical
NextGen Cold Plasma Devices
SurgiHeal Technologies
PlasmaBond Medical Systems
BioPlasma Innovations Inc.
Among these, PlasmaTech Medical Solutions leveraged advanced R&D investments to launch hybrid cold plasma bio welding devices in 2025, capturing a 12% incremental market share within one year. BioWeld Innovations implemented strategic partnerships with premier healthcare providers in Europe, boosting their annual revenue by 22% in 2026. NeoWeld Technologies focused on cost-efficiency improvements through in-house manufacturing, reducing production costs by 15%, which allowed aggressive pricing across emerging markets.
Cold Plasma Bio Welding Device Market Future Outlook
The future of the Cold Plasma Bio Welding Device Market is expected to benefit from the growing demand for minimally invasive and precision surgical techniques. Continued clinical validation and cost optimization are likely to expand use across broader surgical disciplines. Integration with robotic surgery platforms and advanced wound care protocols may further enhance adoption. As healthcare systems emphasize faster recovery and reduced complication rates, cold plasma bio-welding devices are expected to gain increasing relevance.
Cold Plasma Bio Welding Device Market Historical Analysis
The Cold Plasma Bio Welding Device Market developed from advances in plasma physics and minimally invasive surgical technologies. Traditional wound closure methods, such as sutures and staples, often resulted in tissue trauma and prolonged healing. Early experimental studies demonstrated that low-temperature plasma could facilitate tissue bonding without excessive thermal damage. Initial adoption was slow due to limited clinical data and high device costs. However, improvements in plasma control, safety mechanisms, and surgical compatibility gradually increased acceptance, particularly in specialized surgical applications.
Sources
Primary Research Interviews:
Surgeons
biomedical engineers
medical physicists
device developers
hospital procurement managers
Databases:
FDA Medical Device Database
WHO Surgical Technology Data
OECD Health Tech Data
Magazines:
Medical Device Network
Surgical Products Magazine
MedTech Insight
Healthcare Innovation
Biomedical Engineering Today
Journals:
Plasma Medicine Journal
IEEE Transactions on Plasma Science
Journal of Surgical Research
Medical Physics
Advanced Healthcare Materials
Newspapers:
Financial Times (Medical Devices)
Reuters Health
The Guardian (Healthcare)
Bloomberg Medical Technology
The Wall Street Journal (Health)
Associations:
International Society for Plasma Medicine
AdvaMed
IEEE Engineering in Medicine
American College of Surgeons
WHO
Share
Share
About Author
Manisha Vibhute is a consultant with over 5 years of experience in market research and consulting. With a strong understanding of market dynamics, Manisha assists clients in developing effective market access strategies. She helps medical device companies navigate pricing, reimbursement, and regulatory pathways to ensure successful product launches.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients