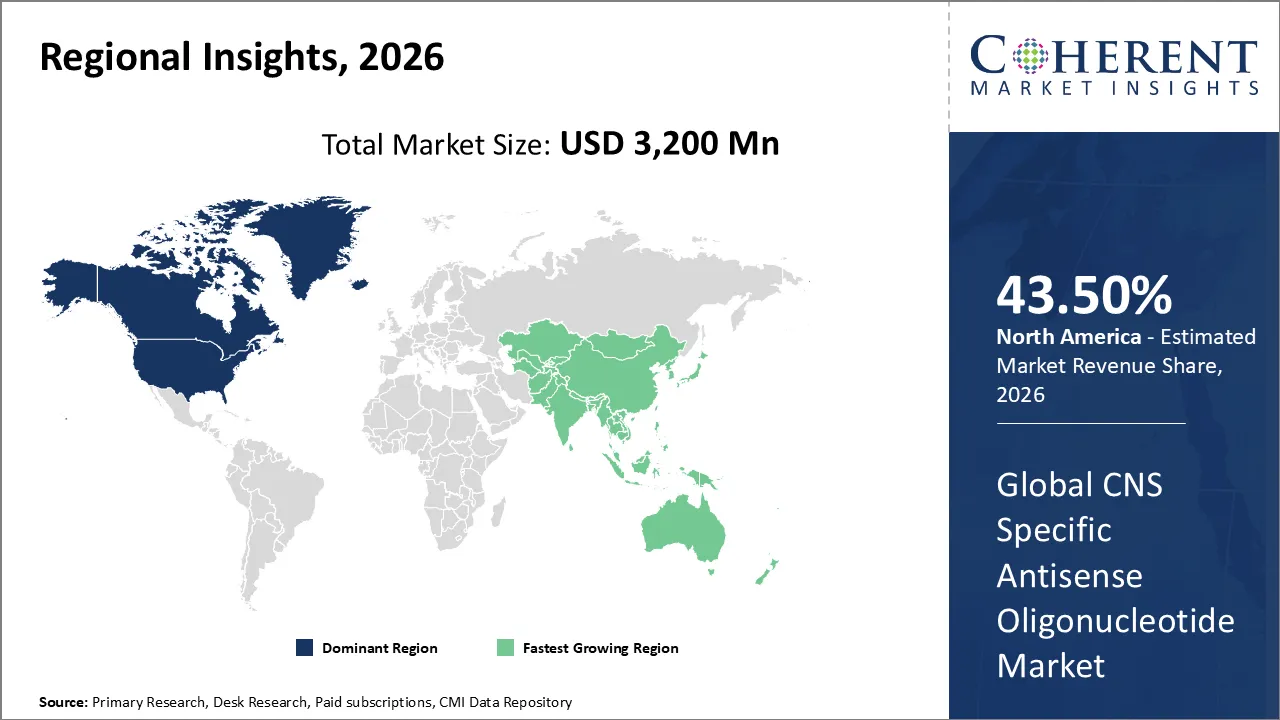
The CNS Specific Antisense Oligonucleotide Market is estimated to be valued at USD 3,200 Mn in 2026 and is expected to reach USD 6,100 Mn by 2033, growing at a compound annual growth The rate (CAGR) of 9.8% from 2026 to 2033.
The Central Nervous System (CNS) Specific Antisense Oligonucleotide Market is progressing rapidly due to the growing applications of precision medicine and treatment of complex neurodegenerative disorders. The increasing number of cases of Spinal Muscular Atrophy (SMA), Huntington’s disease, and Amyotrophic Lateral Sclerosis (ALS), along with the favorable regulatory environment for orphan drugs, is contributing to the growth of the market. These synthetic single-stranded nucleic acid polymers represent a fundamental breakthrough in the biopharmaceutical sector. This is due to their high target specificity and their unique ability to bypass traditional undruggable protein targets by modulating gene expression at the mRNA level.
|
Current Event |
Description and the Impacts |
|
Regulatory Advancements in ASO Therapeutics |
|
|
Technological Innovations in Antisense Oligonucleotide Delivery |
|
|
Epidemiological and Clinical Developments in Target Indications
|
|
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
In terms of drug, the approved segment contributes the highest share of 66.20% in the market in 2026. This is due to the high commercial performance from established therapies that have already received regulatory clearance. These drugs have clear reimbursement pathways and high patient adherence rates compared to those that are currently in clinical trials. The major pharmaceutical companies are focusing on lifecycle management for these products, such as expanding their use to broader patient groups or developing improved formulations. The high expenses of these specialized biologics ensure that they capture the majority of market value. The safety and efficacy profiles of approved drugs provide a level of clinical certainty that attracts steady investment and confidence from healthcare providers.
In terms of indication, the spinal muscular atrophy segment contributes the highest share of 53.60% in 2026 of the market. This is driven by the early and successful application of antisense technology to treat this rare genetic condition. The factor that propels the growth of the market is the extensive use of newborn screening programs for early testing and subsequent treatment for permanent nerve damage. The lack of rational alternatives for this disorder makes these genetic medicines the only ideal option for long-term disease management. The continued research into better dosing schedules and higher concentrations for older patients also solidifies this segment. The high prevalence of the condition relative to other targeted rare diseases ensures a stable and growing base of patients requiring lifelong therapy.
For instance, in June 2025, Biogen Inc. announced the top-line data from the Phase 1 study of salanersen (BIIB115/ION306), an antisense oligonucleotide (ASO) being developed for the treatment of spinal muscular atrophy (SMA). Salanersen has the potential to be highly effective and requires administration only once a year for SMA.
In terms of distribution channel, the hospital pharmacy segment contributes the highest share of 54.30% in 2026 of the market because most central nervous system therapies require complex administration methods, such as injections directly into the spinal canal. These procedures must be performed by trained specialists in a controlled clinical setting, which makes hospitals the central hub for treatment. The specialized storage facilities, which are available at major hospitals. Hospital pharmacies act as a platform to connect neurologists, surgeons, and insurers for patients to receive comprehensive care. All these aspects make hospitals the gateway for the distribution of advanced genetic medicines.
To learn more about this report, Download Free Sample
North America has remained the dominant region with 43.50% in 2026 of the global CNS Specific Antisense Oligonucleotide Market over the past decade. The growth is due to its mature biotechnology infrastructure and substantial presence of pharmaceutical companies. The region offers a favorable regulatory system. The agencies like the FDA provide pathways for organ drugs targeting rare neurological conditions. The development activities are based on addressing high unmet needs such as ALS and Huntington's disease. The presence of cutting-edge healthcare systems ensures that patients have greater access to complex intrathecal administration procedures required for these therapies.
For instance, Biogen Inc. and Alcyone Therapeutics have entered into a licensing and partnership agreement to collaborate on Alcyone's ThecaFlex DRx™ System. This implantable medical device is designed to deliver antisense oligonucleotide (ASO) medicines into the intrathecal space.
The Asia Pacific region is the most rapidly evolving market for CNS ASOs. This is driven by high investments in healthcare modernization across China, Japan, and India. This industry is influenced by a large and aging population, which has led to an increased clinical focus on age-related neurodegenerative disorders. The governments in the region are promoting precision medicine initiatives and are also focusing their genomic research efforts to integrate advanced biopharmaceutics into national health programs. Additionally, the territory is emerging as a global hub for the contract manufacturing and synthesis of high-purity oligonucleotides. As regional biotech firms accelerate their proprietary clinical trials, the area is transitioning from a manufacturing provider to an innovation leader.
The US currently serves as the primary engine of the market and generates the highest revenue in the region. The growth is driven by the high prevalence of neurodegenerative disorders such as Alzheimer's disease and ALS, substantial investments in research and development, and a supportive regulatory environment characterized by key FDA approvals. In addition to this, the presence of major companies like Biogen and Ionis Pharmaceuticals fosters innovation.
For instance, in August 2024, Denali Therapeutics Inc. announced that nonclinical data will be published in Science Translational Medicine. The data will demonstrate that the Oligonucleotide Transport Vehicle (OTV) platform is capable of delivering antisense oligonucleotides (ASOs) to various tissues in the central nervous system (CNS), as well as in skeletal and cardiac muscle, following intravenous administration.
The China CNS-specific antisense oligonucleotide market is an emerging sector with significant growth potential, though it is still in its early stages compared to global counterparts. This growth is propelled by extensive research and development activity, along with robust government support. Currently, the primary focus areas in China include digestive and infectious diseases, while neurological diseases are becoming an area of increasing interest.
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2025 | Market Size in 2026: | USD 3,200 Mn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2026 To 2033 |
| Forecast Period 2026 to 2033 CAGR: | 9.8 % | 2033 Value Projection: | USD 6,100 Mn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Alnylam Pharmaceuticals Inc., Sarepta Therapeutics Inc., Biogen Inc., Ionis Pharmaceuticals Inc., Wave Life Sciences Ltd., Stroke Therapeutic Inc., Dynacure, ProQR Therapeutics N.V., and Q-STATE BIOSCIENCES, INC. |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
The rising global burden of neurological disorders is a primary driver of the Central Nervous System (CNS) Specific Antisense Oligonucleotide (ASO) market. The key factors include the high prevalence of rare genetic diseases, such as Spinal Muscular Atrophy (SMA), the growing aging population that is susceptible to conditions like Alzheimer's and Parkinson's, and significant advancements in research and development. Additionally, drug delivery methods that can bypass the blood-brain barrier are contributing to the market's growth. Currently, North America leads the market, while the Asia Pacific region is expected to experience the fastest growth, highlighting a global effort toward developing novel therapeutic solutions for currently incurable neurological conditions.
For instance, in January 2025, Atalanta Therapeutics has announced two key programs targeting rare neurological illnesses that currently lack FDA-approved treatments. The Boston-based company also reported raising USD 97 million to advance these programs into clinical trials and support the development of additional CNS therapies.
The CNS-specific antisense oligonucleotide (ASO) market is evolving into a strategically important segment of the broader RNA therapeutics landscape, supported by measurable progress in clinical development, regulatory validation, and technology innovation. Neurological disorders represent one of the largest unmet medical needs globally, with over one billion people affected by CNS conditions according to international health agencies, reinforcing the long-term demand for targeted genetic therapies.
Data from industry and clinical registries indicate that more than 50 CNS-focused ASO candidates are currently active across clinical development stages, with neurological indications accounting for an estimated one-third of the global antisense pipeline. Rare and genetically defined disorders such as spinal muscular atrophy, Huntington’s disease, and certain inherited ataxias remain primary focus areas, though pipeline expansion into Alzheimer’s disease, Parkinson’s disease, and epilepsy is increasingly evident.
Technological progress in RNA chemistry modifications and intrathecal delivery approaches has improved target specificity, durability of response, and central nervous system exposure, strengthening clinical confidence. Additionally, sustained growth in registered CNS-related clinical trials, alongside rising participation from specialized CDMOs and academic research centers, reflects increasing institutional investment.
Definition: The CNS Specific Antisense Oligonucleotide Market includes the development, manufacturing, and sale of synthetic single-stranded nucleic acid sequences for the treatment of neurological and neurodegenerative diseases. These therapies function by binding to specific messenger RNA (mRNA) sequences within the Central Nervous System, thereby silencing disease-causing genes or modulating protein expression at the molecular level. Because ASOs cannot cross the blood barrier, this market is characterized by specialized delivery methods, such as intrathecal administration. The key drivers include the rising prevalence of conditions alongside rapid advancements in nucleotide chemistry that enhance drug stability and precision.
Share
Share
About Author
Vipul Patil is a dynamic management consultant with 6 years of dedicated experience in the pharmaceutical industry. Known for his analytical acumen and strategic insight, Vipul has successfully partnered with pharmaceutical companies to enhance operational efficiency, cross broader expansion, and navigate the complexities of distribution in markets with high revenue potential.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients