Global cluster headache market is estimated to be valued at USD 449.3 Mn in 2025 and is expected to reach USD 694.0 Mn by 2032, exhibiting a compound annual growth rate (CAGR) of 6.4% from 2025 to 2032. Cluster headache is a neurological disorder characterized by severe pain on one side of the head. Currently available treatment options for this disease provide only symptomatic relief.
Discover market dynamics shaping the industry: Download Free Sample
The market growth is driven by increasing prevalence of cluster headaches and the need for more effective therapeutics. Many biopharmaceutical companies are actively researching novel therapies such as monoclonal antibodies and calcitonin gene-related peptide (CGRP) antagonists to provide improved treatment outcomes.
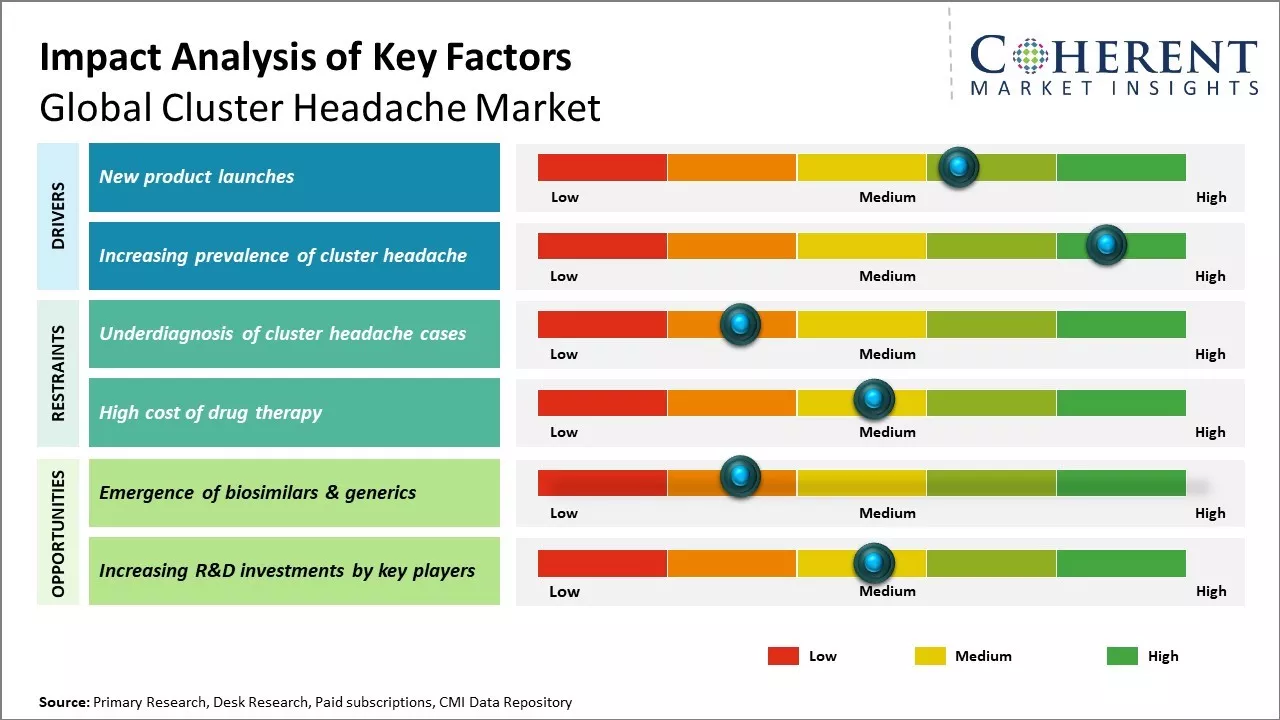
New Product Launches
Global cluster headache market has witnessed significant growth due to numerous new product launches that have expanded treatment options for people with cluster headache. Pharmaceutical companies have invested heavily in developing novel drug delivery mechanisms and medications that target cluster headaches in new ways. This increased focus on innovation has resulted in more effective acute and preventative therapies. Some of the major new product categories to emerge include novel oral treatments, nerve stimulators, and injectable medications. Oral treatments such as ditans and lasmiditan provide acute options beyond traditional triptans, allowing patients to avoid potential drug interactions or side effects. Sprays such as zolmitriptan nasal have also extended non-oral dosing possibilities. Preventative treatments have expanded. The U.S. FDAapproved the first dedicated monoclonal antibody galcanezumab-gnlm in 2020, according to the Food and Drug Administration.
Meanwhile, non-drug devices like the gammaCore Sapphire have provided new hope to patients who find oral medications insufficient through non-invasive vagus nerve stimulation.
Get actionable strategies to beat competition: Download Free Sample
Increasing Prevalence of Cluster Headache
Cluster headache is a severe primary headache disorder that is characterized by intense pain on one side of the head. There has been increase in cases of this disease due to greater awareness and accurate diagnosis of the condition. Earlier, cluster headache was often misdiagnosed as migraine or sinus headache due to its excruciating nature localized to one side of the head. However, with enhancement in doctors' understanding of its distinctive symptoms like periodic attacks, Horner's syndrome, and others, more patients with actual cluster headache are being identified now. Moreover, improved medical surveillance and longitudinal epidemiological research have enabled getting a clearer picture of how widespread this condition is. Several population-based studies conducted in the recent decades across countries like U.S., U.K. Canada, Italy, Norway, and others have estimated the point prevalence of cluster headache to be anywhere between 50 to 300 per 100,000 persons. The higher prevalence numbers have been reported from Norway, suggesting a possible genetic role. The disease mainly affects the adult population between 20-50 years of age with a significant male predisposition. For Instance In April 2020, according to an research article published by BMC (BioMed Central), a systematic review and meta-analysis was conducted following the guidelines of the preferred reporting items for systematic reviews and meta-analyses protocols (PRISMA-P). The study revealed that approximately 6.27% of patients with cluster headache had a family history, with an overall I2 of 73%.
Key Takeaways from Analyst:
Global cluster headache market is expected to witness significant growth over the forecast period. Due to debilitating nature of cluster headaches and lack of FDA approved treatments, the market remains largely underpenetrated. North America currently dominates the market, owing to higher awareness and rapid adoption of emerging therapies. However, Asia Pacific is likely to emerge as the fastest growing region with China and India expected to triple the patient pool.
Lack of approved drugs and underdiagnosis in developing regions can hamper the market growth. Furthermore, high costs of emerging CGRP therapies can hamper the market growth in developing countries. Rising access to diagnostic techniques provides an opportunity to enhance diagnosis rates globally. Increased healthcare expenditure in Asia Pacific bodes well for market expansion.
Growing research on pathophysiology also promises targeted treatment approaches. Favorable reimbursement for cluster headache treatment in the U.S. and EU can drive the market growth. Furthermore, companies investing in pediatric applications can gain first-mover advantage. However, stiff competition from generic manufacturers may impede premium pricing of novel therapies. Overall, the market remains ripe for future growth subject to effective management of risks.
Market Challenges: Underdiagnosis of Cluster Headache Cases
Underdiagnosis of cluster headache cases can hamper the cluster headache market growth. Cluster headache is often misdiagnosed or underdiagnosed, which delays appropriate treatment. Cluster headache is known as the most painful headache condition but it remains heavily under-diagnosed. The attacks are comparatively short but occur frequently and at irregular intervals. This confusing pattern often leads to misdiagnosis of cluster headache as migraine or sinusitis. Furthermore, the attacks are severe enough to disable the patient temporarily, so these may not report to a doctor during every attack due to the excruciating pain. This abnormal rhythm of attacks can easily mislead physicians unaware of cluster headache. Moreover, cluster headache is much rarer than other headache conditions like migraine or tension headaches. General physicians and neurologists may not consider it in the differential diagnosis leading to wrong prescriptions.
Market Opportunities: Emergence of Biosimilars & Generics
The emergence of biosimilars and generic drugs can offer opportunities for cluster headache market growth. Biosimilars are biologic medical products that are highly similar to an already FDA-approved biologic drug (reference product). Given their comparable quality, safety and efficacy to the reference product, but at a lower cost, biosimilars allow more patients to access effective treatment options. As cluster headaches are relatively rare conditions requiring specialty medications, increasing biosimilar availability could make treatments more affordable and accessible to patients. Several blockbuster drug patents are set to expire in the next 2-3 years, opening pathways for generic versions. This includes notable cluster headache drugs like erenumab and galcanezumab, monoclonal antibodies targeting calcitonin gene-related peptide. When the patents expire, multiple generic manufacturers can produce lower-cost alternatives, generating market competition. According to the World Health Organization, the introduction of generic medicines in global markets led to a total saving of over US$ 1.5 trillion in global health expenditure between 2000 to 2016. As generics capture a larger market share post-patent expiry, these have the potential to significantly reduce medication costs for patients as well as whole health systems.
Discover high revenue pocket segments and roadmap to it: Download Free Sample
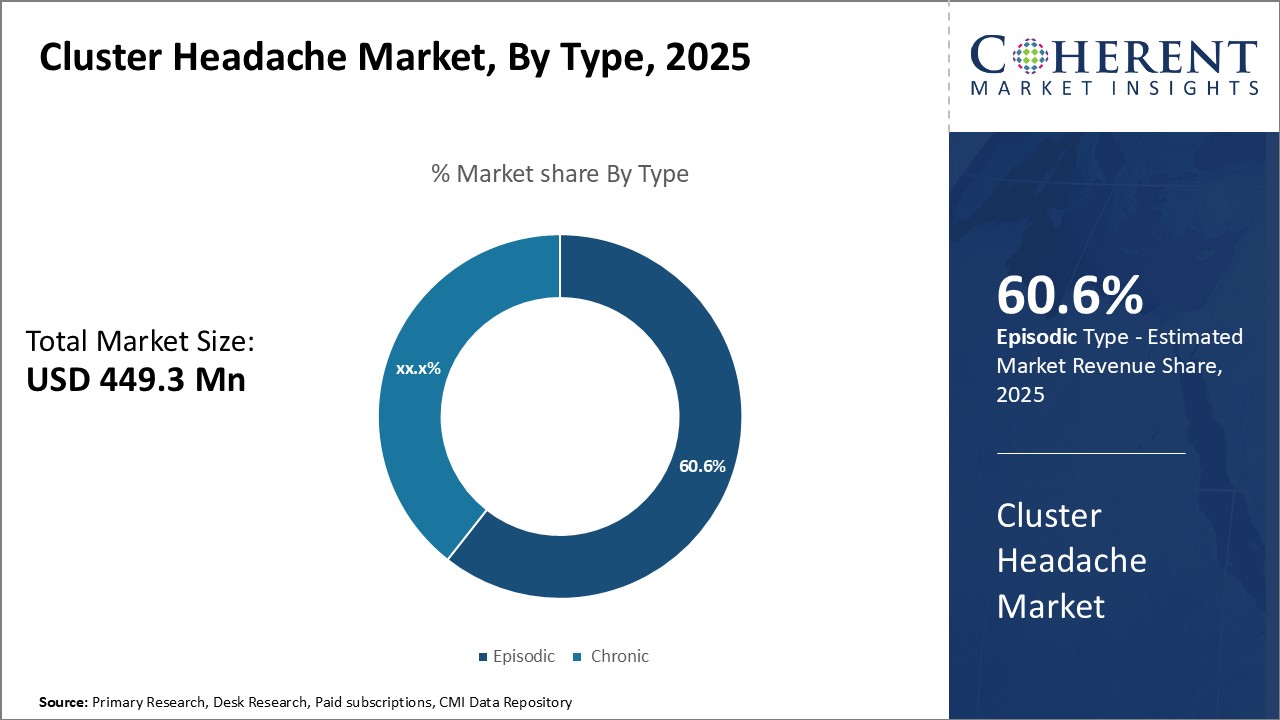
Insights, By Type - Convenient treatment drives growth of episodic segment
In terms of type, episodic segment is estimated to contribute the highest market share of 60.6% in 2025, owing to the convenient treatment options available. Episodic cluster headaches occur in regular clusters over time with remission periods in between. Since the timing of episodic headaches is predictable to some extent, these allow patients and doctors to plan treatment approaches accordingly. Fast-acting rescue medications play a major role in aborting individual episodic cluster headache attacks. The ease of use and on-demand administration of triptans and oxygen therapy makes them preferred first-line treatment options. Their ability to provide quick relief within 15-30 minutes, if taken at the right time, has made episodic cluster headaches relatively manageable. Preventive therapies are not required on a daily basis and can be initiated only during an active headache period. This episodic nature makes cluster headaches less disruptive to daily lives as compared to chronic form. The short duration and defined cycles of episodic cluster headaches allow screening and testing of various acute and preventive therapies to ascertain their efficacy.
Insights, By Drug Type - Fast-acting medications drive growth of triptan segment
In terms of drug type, fast-acting drugs segment is estimated to contribute the highest market share of 40.21 % in 2025, due to the unmet need for quick relief. Cluster headaches are characterized by excruciating pain that makes patients incapable of performing normal activities or remaining motionless. This debilitating nature requires medications that can arrest headaches within a very short timeframe. Among available pharmacological options, triptans (serotonin 5-HT1B/1D receptor agonists) have emerged as the most effective acute medications due to their ultra-fast action. Sumatriptan was the first triptan introduced and still remains the market leader due to its ability to relieve pain within 15-30 minutes for majority of patients. Other derivatives like zolmitriptan and naratriptan have comparable onset of action. Their high efficacy rates of over 60.5% along with little to no adverse effects have created a strong place for triptans as the frontline treatment for cluster headache attacks.
Insights, By Route of Administration - Convenient administration boosts oral route dominance
In terms of route of administration, oral segment is estimated to contribute the highest market share of 60.12 % in 2025, due to its dosing convenience. Cluster headaches are intermittent yet unpredictable in nature, often striking patients without warning. This poses challenges for administration of acute treatment at the right time. Oral formulations like tablets/capsules allow pain-stricken patients to self-administer rescue medications discreetly and independently without depending on caregivers. Their portability and easy storage as solid dosage forms add to the practicability of oral formulations. Oral triptans like sumatriptan and zolmitriptan have demonstrated high bioavailability.. With rapid absorption through gastrointestinal tract, these can exert pain-relieving effects within 15-30 minutes of oral intake. The avoidance of injection procedures and medical expertise for administration increase treatment adherence with oral route. These merits have made oral formulations highly acceptable as the first-line treatment approach.
Need a Different Region or Segment? Download Free Sample
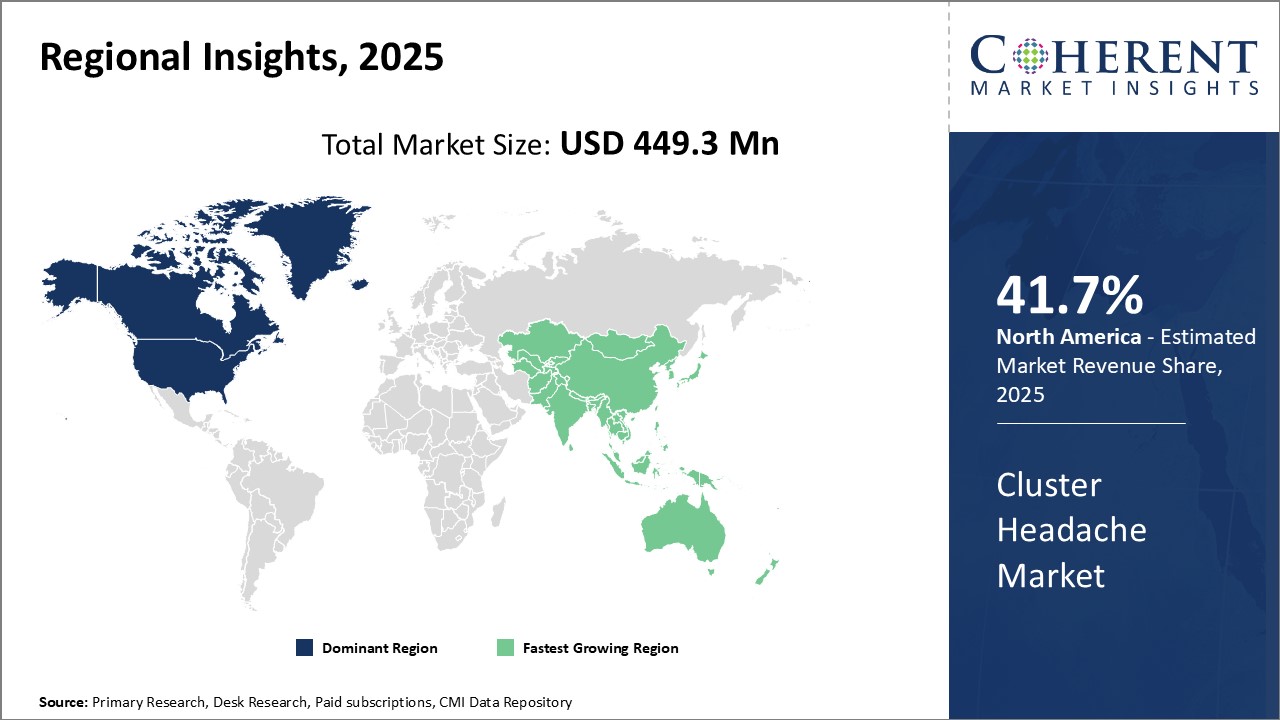
North America has dominated the global cluster headache market for several years, and is estimated to hold market share of 41.7% in 2025. The region has a highly developed healthcare infrastructure and higher healthcare spending. Many global pharmaceutical companies have their headquarters in countries like the U.S. that provides them early access to new and innovative drug therapies.
There is also strong industry presence with several pharmaceutical companies focusing significant resources on R&D for cluster headache therapies. This has resulted in the early availability of many leading drugs for preventive and acute treatment of cluster headaches. The pricing and insurance coverage of such drugs are favorable in the region, allowing for higher patient adoption rates compared to other regions.
Asia Pacific region has emerged as the fastest growing market for cluster headache. Rapid economic growth and development of healthcare systems have enhanced accessibility and affordability of treatments. Countries like China and India offer huge market opportunities due to their large population bases experiencing cluster headache.
Cluster Headache Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 449.3 Mn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 6.4% | 2032 Value Projection: | USD 694.0 Mn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
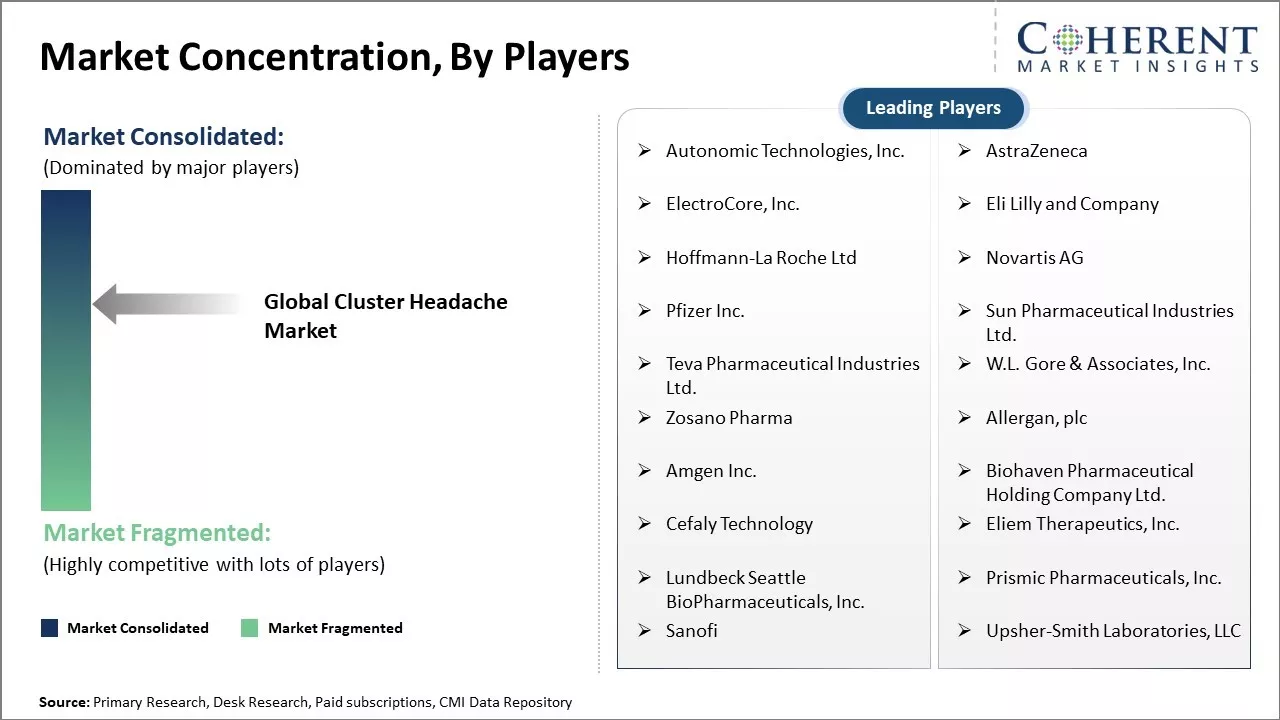
Autonomic Technologies, Inc., Epic Systems Corporation, AstraZeneca, ElectroCore, Inc., Eli Lilly and Company, Hoffmann-La Roche Ltd, Novartis AG, Pfizer Inc., Sun Pharmaceutical Industries Ltd., Teva Pharmaceutical Industries Ltd., W.L. Gore & Associates, Inc., Zosano Pharma, Allergan, plc, Amgen Inc., Biohaven Pharmaceutical Holding Company Ltd., Cefaly Technology, Eliem Therapeutics, Inc., Lundbeck Seattle BioPharmaceuticals, Inc., Prismic Pharmaceuticals, Inc., Sanofi, Upsher-Smith Laboratories, LLC |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Share
Share
About Author
Ghanshyam Shrivastava - With over 20 years of experience in the management consulting and research, Ghanshyam Shrivastava serves as a Principal Consultant, bringing extensive expertise in biologics and biosimilars. His primary expertise lies in areas such as market entry and expansion strategy, competitive intelligence, and strategic transformation across diversified portfolio of various drugs used for different therapeutic category and APIs. He excels at identifying key challenges faced by clients and providing robust solutions to enhance their strategic decision-making capabilities. His comprehensive understanding of the market ensures valuable contributions to research reports and business decisions.
Ghanshyam is a sought-after speaker at industry conferences and contributes to various publications on pharma industry.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients