Clot Management Devices Market Size and Forecast – 2025 – 2032
The Global Clot Management Devices Market size is estimated to be valued at USD 6.15 billion in 2025 and is expected to reach USD 10.92 billion by 2032, exhibiting a compound annual growth rate (CAGR) of 8.2% from 2025 to 2032.
Global Clot Management Devices Market Overview
Clot management devices consist of mechanical thrombectomy systems, aspiration catheters, infusion catheters, vena cava filters, and hemodynamic pumps used in treating DVT, PE, and arterial embolisms. These devices aim to rapidly restore blood flow, reduce reliance on thrombolytics, and minimize bleeding risk. Modern systems emphasize single-session clot removal, improved navigation, and compatibility with minimally invasive vascular interventions.
Key Takeaways
The Cardiovascular Disorders application segment dominates the market, accounting for over 52% share due to escalating cardiac disease prevalence and procedural complexity.
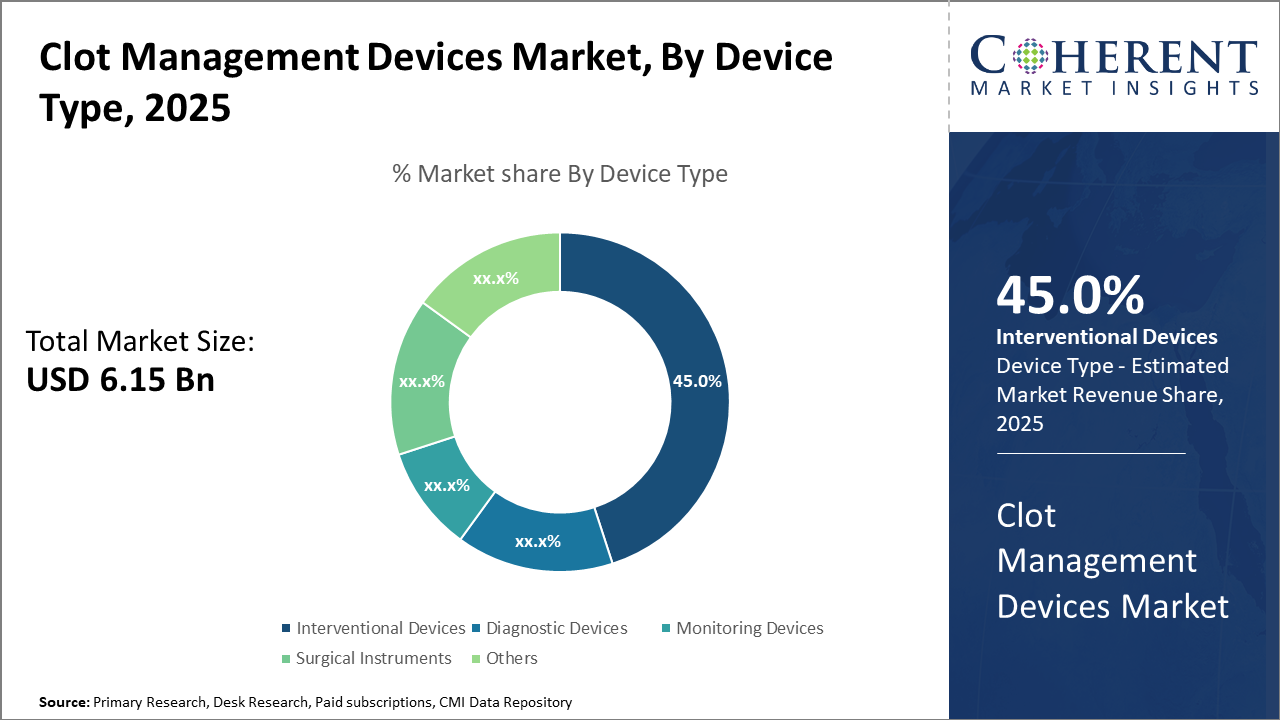
Interventional Devices represent the leading device category with a 45% share, driven by expanding minimally invasive treatment methodologies that cater to acute clot removal needs.
Hospitals remain the largest end-user segment, reflecting the concentration of advanced therapeutic capabilities and procedural volumes.
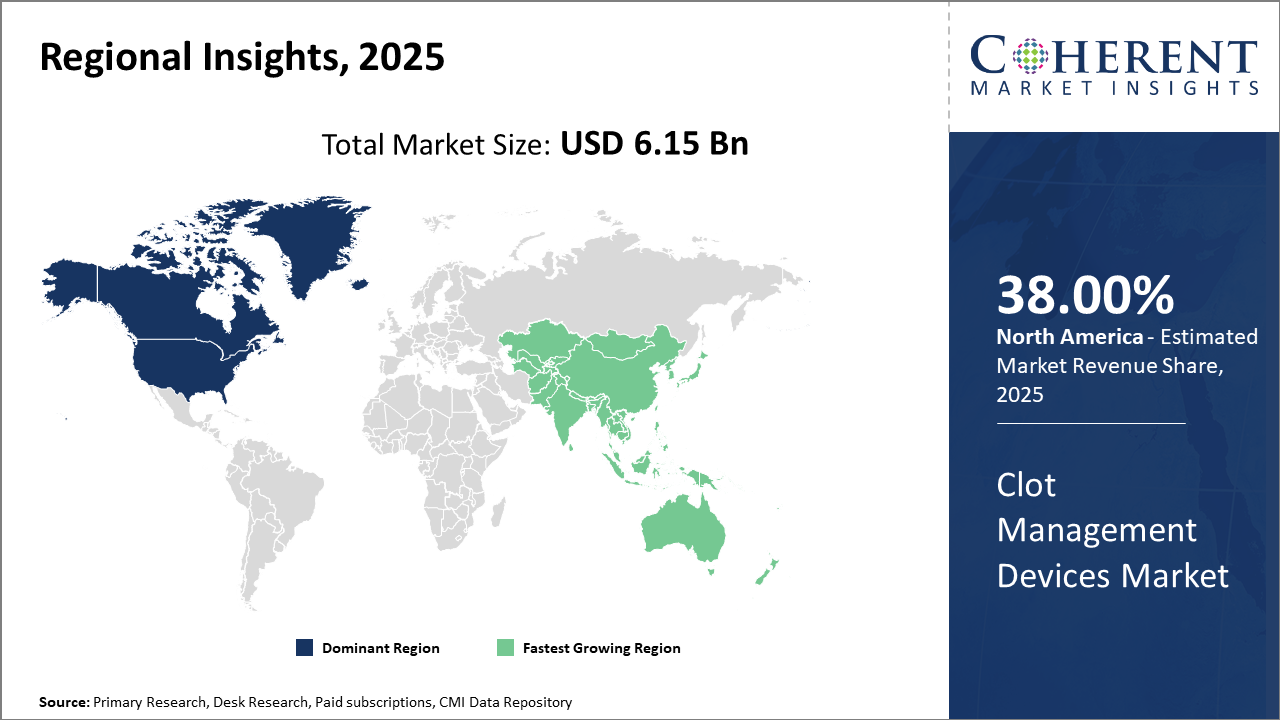
Regionally, North America leads the clot management devices market share owing to its mature healthcare ecosystem, supportive reimbursement structures, and technological innovation hubs.
Asia Pacific is the fastest-growing region with an impressive CAGR fueled by increasing healthcare expenditure, improving infrastructure, and expanding patient awareness.
Europe follows closely with diversified application segments and consistent investment in healthcare innovation.
Clot Management Devices Market Segmentation Analysis
To learn more about this report, Download Free Sample
Clot Management Devices Market Insights, By Device Type
Interventional Devices dominate the market share with 45%. This segment’s dominance is propelled by extensive application in emergency clot removal treatments such as mechanical thrombectomy and catheter-directed thrombolysis, supported by technological advancements that enhance procedural efficacy and safety. The fastest growing subsegment is Monitoring Devices, driven by the rise of wearable and remote monitoring solutions facilitating continuous clot risk assessment outside clinical settings. Diagnostic Devices maintains a significant presence due to evolving imaging technologies that improve clot detection accuracy.
Clot Management Devices Market Insights, By Application
Cardiovascular Disorders are leading with a 52% market share. This leadership is due to the increasing incidence of cardiac-related thrombotic conditions requiring timely intervention using clot management devices. Deep Vein Thrombosis is identified as the fastest growing subsegment, spurred by rising awareness and screening programs in both developed and developing countries. Pulmonary Embolism devices hold a critical niche for acute care, whereas Peripheral Artery Disease devices focus on chronic management solutions with moderate growth.
Clot Management Devices Market Insights, By End-User
Hospitals dominate as the leading segment due to their comprehensive facilities with capabilities for advanced clot management procedures and higher patient throughput. Ambulatory Surgical Centers represent the fastest-growing end-user subsegment, driven by rising demand for outpatient clot removal and monitoring services, facilitated by improvements in procedural techniques. Diagnostic Laboratories play a supporting role focused on clot detection and analysis, while Home Care Settings are witnessing the gradual adoption of portable monitoring devices catering to chronic patients.
Clot Management Devices Market Trends
The market has undergone substantial transformation driven by technological innovation and demographic transitions.
A salient market trend includes the rising adoption of AI-enhanced diagnostic tools facilitating precision medicine, evidenced by recent integration in over 40% of new diagnostic device launches globally in 2024.
Additionally, the shift towards minimally invasive procedures fuels growth in interventional devices, with mechanical thrombectomy procedures expanding by 28% globally from 2023 to 2025.
Portable monitoring devices enabling remote patient management have also seen accelerated adoption, propelled by the telehealth surge, attaining a 35% increase in unit sales.
Clot Management Devices Market Insights, By Geography
To learn more about this report, Download Free Sample
North America Clot Management Devices Market Analysis and Trends
In North America, the dominance in the Clot Management Devices market arises from its advanced healthcare infrastructure, high per capita healthcare spending, and progressive reimbursement environments that collectively account for over 38% of the global market share. The United States, specifically, benefits from a robust R&D ecosystem and supportive FDA pathways, enabling the quick commercialization of innovative devices. Well-established industry players headquartered in the region contribute extensively to technological advancements and market maturation.
Asia Pacific Clot Management Devices Market Analysis and Trends
Meanwhile, the Asia Pacific exhibits the fastest growth, with a CAGR of 10.1% driven by expanding healthcare infrastructure, increasing hospital capacities, and growing awareness of clot-related diseases in emerging economies such as China and India. Government initiatives focusing on improving cardiovascular care and rising disposable incomes further stimulate market penetration. Noteworthy companies actively enhancing their presence through regional partnerships and localized manufacturing propel this growth trajectory.
Clot Management Devices Market Outlook for Key Countries
USA Clot Management Devices Market Analysis and Trends
The USA's market remains the largest globally due to sustained investments in healthcare and technology innovation. Clinical adoption of novel mechanical thrombectomy devices surged by 33% in 2024, supported by policy frameworks encouraging early intervention in stroke treatment. Prominent players headquartered in the US, including Medtronic and Boston Scientific, continue to dominate market activity through product launches and strategic acquisitions, thus solidifying the country's leadership in market revenue and innovation.
China Clot Management Devices Market Analysis and Trends
China's market for Clot Management Devices is expanding rapidly due to increasing prevalence of thrombotic conditions among aging populations and government-sponsored programs promoting cardiovascular health screening. The country witnessed a 27% rise in usage of diagnostic and interventional clot management devices in 2024, bolstered by local manufacturing initiatives and growing partnerships between global manufacturers and domestic distributors. Such dynamics contribute to China's position as a burgeoning force within the Asia Pacific region.
Analyst Opinion
Enhanced Diagnostic Precision Drives Market Revenue: The surge in adoption of advanced imaging and clot detection devices has significantly expanded the market share of diagnostic subsegments. For example, usage of point-of-care ultrasound devices for clot visualization increased by over 25% in North American hospitals in 2024, highlighting demand-driven market growth and advancing diagnostic accuracy.
Procedural Integration Propels Demand: Interventional clot management devices embedded with enhanced drug-delivery and mechanical thrombectomy features are witnessing elevated demand, propelled by rising minimally invasive cardiac procedures. Data from 2025 hospital records indicate a 30% year-on-year increase in mechanical thrombectomy procedures, reflecting an expanding procedural landscape influencing market dynamics.
Regional Healthcare Investments Influence Market Expansion: North America and Asia Pacific show differentiated supply-side growth trends influenced by healthcare infrastructure upgrades and reimbursement policies. For instance, Medicare’s updated reimbursement guideline for clot management devices in the USA since late 2024 elevated market revenue by 12%, underpinning favorable policy-driven growth.
Market Challenges and Restraints Affect Penetration in Emerging Economies: Despite robust demand, market restraints such as high device costs and regulatory complexities moderate penetration rates in Latin America and Africa. Recent regulatory amendments in Brazil in 2025 seek to streamline approvals but device affordability remains a constraint limiting overall market growth.
Market Scope
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2025 | Market Size in 2025: |
USD 6.15 billion |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 8.2% | 2032 Value Projection: |
USD 10.92 billion |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: | Medtronic, Boston Scientific Corporation, Abbott Laboratories, Terumo Corporation, Becton Dickinson and Company (BD), Edwards Lifesciences, Siemens Healthineers, Philips Healthcare, Johnson & Johnson, Stryker Corporation, BioMedix, Penumbra Inc. | ||
| Growth Drivers: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Clot Management Devices Market Growth Factors
The increased prevalence of cardiovascular and thromboembolic diseases remains the foremost growth driver, supported by global aging demographics and lifestyle shifts. Data from the American Heart Association indicates a 5% annual rise in stroke incidence among adults above 65 since 2023, underscoring a growing patient base requiring clot management solutions. Secondly, advancements in diagnostic imaging coupled with real-time monitoring devices enhance early clot detection, significantly improving patient outcomes and driving adoption across healthcare settings.
Furthermore, rising investments by governments, especially in North America and the Asia Pacific, into healthcare infrastructure and reimbursement incentives for clot-related procedures tailor favorable market environments. Finally, the trend toward minimally invasive therapies encourages the use of technologically sophisticated interventional devices, fostering innovation cycles and commercial uptake, as evidenced by a 20% increase in minimally invasive clot removal procedures logged worldwide in 2024.
Clot Management Devices Market Development
In January 2024, Stryker launched the NeuroThrive Thrombectomy System, introducing a next-generation mechanical thrombectomy solution designed to improve clot engagement and extraction efficiency in acute ischemic stroke procedures. The platform features an optimized delivery profile, enhanced aspiration compatibility, and improved device flexibility to allow smoother navigation through tortuous cerebral vessels.
In 2019, Medtronic launched the Solitaire™ X revascularization device as the latest advancement in it’s mechanical thrombectomy portfolio, which is engineered to deliver more efficient and reliable clot retrieval for patients experiencing acute ischemic stroke. This next-generation stent retriever incorporates an enhanced nitinol construction with improved radial force and clot-integration capability, allowing for stronger engagement with both hard and fibrin-rich thrombi.
Key Players
Leading Companies of the Market
Medtronic
Boston Scientific Corporation
Abbott Laboratories
Terumo Corporation
Becton, Dickinson and Company (BD)
Edwards Lifesciences
Siemens Healthineers
Philips Healthcare
Johnson & Johnson
Stryker Corporation
BioMedix
Penumbra Inc.
Several of these market players have adopted aggressive growth strategies such as alliances and continuous R&D investments. For instance, Medtronic’s strategic acquisition in 2024 of a leading thrombectomy device innovator allowed an expanded product portfolio targeting ischemic stroke treatment; this led to a 15% increase in its clot management device revenue by Q3 2025. Similarly, Boston Scientific’s partnership with regional distributors in Asia Pacific has accelerated market penetration, resulting in localized devices compliant with regional regulatory frameworks and a 22% increase in sales in India and China combined during 2024.
Clot Management Devices Market Future Outlook
The market will continue evolving toward faster, safer, and more portable interventions. Expect single-session systems with improved clot-retrieval efficiency, integrated aspiration and imaging compatibility, and devices optimized for peripheral venous thromboembolism (PE/DVT) in addition to neurovascular use. Development of thrombus-specific coatings and adjunct pharmacomechanical techniques could reduce adjunct lytic doses and bleeding risk. Tele-hub models and mobile stroke units will increase demand for compact, easy-deploy systems. Reimbursement and stroke system organization will remain crucial—improved outcomes data showing reduced disability and cost-savings will accelerate procurement at regional stroke centers.
Clot Management Devices Market Historical Analysis
Clot management advanced from systemic thrombolysis and surgical embolectomy to minimally invasive, catheter-based solutions. Early thrombolytic therapy reduced mortality but carried bleeding risks; the late 20th century introduced catheter-directed thrombolysis and mechanical thrombectomy devices. Stroke care particularly catalyzed innovation, as stent-retriever and aspiration systems demonstrated superior outcomes for large-vessel occlusion, establishing mechanical thrombectomy as standard of care. The industry progressively optimized catheter flexibility, clot engagement mechanisms, and aspiration pumps to improve first-pass success and reduce distal embolization. Parallel improvements in imaging and neurointerventional training supported broader adoption in stroke centers.
Sources
Primary Research Interviews:
Interventional neurologists
Vascular surgeons
Cath lab nurses
Device design engineers
Databases:
PubMed Vascular Intervention Literature
ClinicalTrials.gov (thrombectomy trials)
FDA device approvals database
WHO Stroke Data Repository
Magazines:
NeuroInterventional Today
Vascular Specialist
Cath Lab Digest
Interventional Cardiology Today
Journals:
Stroke
Journal of NeuroInterventional Surgery
Journal of Vascular and Interventional Radiology (JVIR)
Circulation
Newspapers:
The Wall Street Journal (Health)
The Guardian (Science)
The Hindu (Health)
Financial Times (Healthcare)
Associations:
American Heart Association (AHA)
Society of NeuroInterventional Surgery (SNIS)
European Society of Minimally Invasive Neurological Therapy (ESMINT)
Society of Interventional Radiology (SIR)
Share
Share
About Author
Komal Dighe is a Management Consultant with over 8 years of experience in market research and consulting. She excels in managing and delivering high-quality insights and solutions in Health-tech Consulting reports. Her expertise encompasses conducting both primary and secondary research, effectively addressing client requirements, and excelling in market estimation and forecast. Her comprehensive approach ensures that clients receive thorough and accurate analyses, enabling them to make informed decisions and capitalize on market opportunities.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients