Clostridioides Difficile Infection Treatment Market is estimated to be valued at USD 1,024.9 Mn in 2025 and is expected to reach USD 1,659.8 Mn in 2032, exhibiting a compound annual growth rate (CAGR) of 7.13% from 2025 to 2032. Clostridiodes Difficile Infection is a germ that infects the inner lining of the colon and causes diarrhea and colitis (an inflammation of the colon). The disturbance of normal, healthy bacteria in the colon, frequently as a result of antibiotics, leads to Clostridium difficile colitis. Additionally, spores can spread C. difficile from one person to another. The colon may suffer serious harm, and it might even be fatal. Antibiotics are used in treatment of the clostridioides infection. The infection may return despite antibiotic treatment. Surgery or a faecal transplant may be required in rare circumstances.
Analysts’ Views on Global Clostridioides Difficile Infection Treatment Market:
The clostridioides difficile infection treatment market growth can be driven by the increasing approval of the drug products by the regulatory bodies for the treatment of Clostridioides difficile infection. For instance, in November 2022, Ferring Pharmaceuticals, a pharmaceutical company, announced that Rebyota, the fecal microbiome therapy, received the U.S. Food and Drug Administration approval for the prevention of the recurrence of Clostridioides difficile infection.
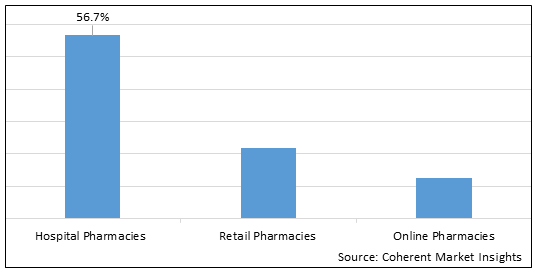
Figure 1. Global Clostridioides Difficile Infection Treatment Market Share (%), by Distribution Channel, 2025
To learn more about this report, Download Free Sample
Global Clostridioides Difficile Infection Treatment Market - Driver
Rising prevalence of clostridioides difficile infection
The rising prevalence of clostridioides difficile infection is expected to drive growth of the global clostridiodes difficile infection treatment market over the forecast period. For instance, in August 2022, according to the data published by the National Center for Biotechnology Information, around 453, 000 cases of clostridioides difficile infection were estimated in the year 2021 in the U.S. Furthermore, according to the data published by the Centers for Disease Control and Prevention, it was estimated that in the U.K., the annual incidence of clostridioides difficile infection (Clostridioides Difficile Infection) was 22.2 per 100,000 population in the year 2021.
Clostridioides Difficile Infection Treatment Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 1,024.9 Mn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 7.13% | 2032 Value Projection: | USD 1,659.8 Mn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Novartis AG, Baxter, ANI Pharmaceuticals, Inc., Mylan N.V., Akorn, Sun Pharmaceutical Industries Ltd., Merck & Co., Inc., B. Braun Medical Inc., Teva Pharmaceutical Industries Ltd., Hikma Pharmaceutical PLC, Perrigo Pharmaceutical., Apotex Inc., AbbVie Inc., Ferring Pharmaceuticals, Fresenius Kabi USA., Pfizer Inc., Strides Pharma Science Limited., Sanofi., AstraZeneca., Eli Lilly and Company., Actelion Pharmaceuticals Ltd., and Astellas Pharma |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Increasing research and development activities for prevention of the clostridioides difficile infection
The increasing research and development activities by the key players in the market for launching new treatment therapy or vaccines for preventing clostridioides difficile infection is expected to drive growth of the global clostridiodes difficile infection treatment market. For instance, in March 2022, Pfizer Inc., a pharmaceutical company, announced the results from the Phase 3 CLOVER trial (Clostridium Difficile Vaccine Efficacy Trial). Although the trial did not achieve its pre-specified primary aim of prevention of Clostridioides Difficile Infection, initial assessments of two protocol-defined secondary endpoints revealed a highly favorable impact in lowering Clostridioides Difficile Infection severity and 100% immunisation efficacy in preventing medically attended Clostridioides Difficile Infection. The investigational vaccination was safe and well tolerated, according to safety reviews.
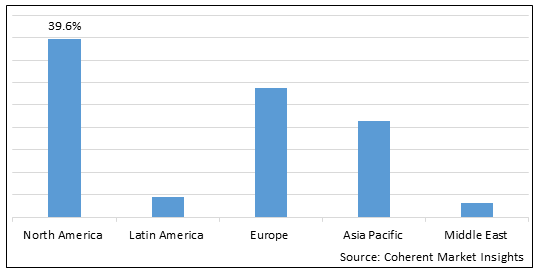
Figure 2. Global Clostridioides Difficile Infection Treatment Market Share (%), by Region, 2025
To learn more about this report, Download Free Sample
Global Clostridioides Difficile Infection Treatment Market - Regional Analysis
Among all regions, North America is expected to dominate the market over the forecast period. This is attributed due to North America holding 39.6% market share in the year 2025 and market players operating in the region focusing on carrying out research and development activities for launching new therapy for the treatment of clostridioides difficile infection. For instance, in September 2022, Seres Therapeutics, Inc., a biotechnology company, announced the completion of the rolling Biologics License Application (BLA) submission to the U.S. Food and Drug Administration (FDA) for SER-109 for the prevention of recurrent C. difficile infection (rClostridioides Difficile Infection). Due to SER-109's U.S. Food and Drug Administration (FDA) Breakthrough Therapy designation, the Biologics License Application (BLA) may receive priority review. If granted, Seres estimates SER-109's potential approval and release in the first half of 2023, with SER-109 possibly becoming the first oral microbiome treatment ever to receive the U.S. Food and Drug Administration (FDA) approval.
Europe is expected to be the second-largest region over the forecast period, due to increasing clinical trials for treating the clostridioides difficile infection. For instance, in March 2022, Lausanne University Hospital, a University hospital in Lausanne, Switzerland initiated a clinical trial “to evaluate the efficacy of fecal microbiota transplantation (FMT) versus the pragmatic use of the standard of care treatment (either vancomycin or fidaxomicin) in severe and non-severe first recurrence of Clostridioides difficile infection (rClostridioides Difficile Infection)”. The fecal microbiota transplantation versus vancomycin or fidaxomicin in clostridioides difficile infection study is currently in the phase 3 trial. The study is estimated to be completed by December 2025.
Global Clostridioides Difficile Infection Treatment Market – Impact of Coronavirus (COVID-19) Pandemic
Since the COVID-19 virus outbreak in December 2019, the disease has spread to over 100 countries across the globe and the World Health Organization declared it a public health emergency on January 30, 2020.
COVID-19 can affect the economy in three main ways: by directly affecting the production and demand of drugs, by creating disruptions in distribution channels, and through its financial impact on firms and financial markets. Due to nationwide lockdowns, several countries such as China, India, Saudi Arabia, the U.A.E., Egypt, and others are facing problems with regard to the transportation of drugs from one place to another.
However, the COVID-19 pandemic had a positive impact on the global clostridioides difficile infection treatment market, owing to the increased prevalence of the clostridioides difficile infection during the pandemic, which raised demand for drugs required in the treatment of the clostridioides difficile infection. For instance, in August 2020, according to the data published by the National Center for Biotechnology Information, there are considerable concerns regarding a potential rise in Clostridioides difficile infections (Clostridioides Difficile Infection s), particularly in the elderly population, as a consequence of the extensive use of broad-spectrum antibiotics during the COVID-19 pandemic. Due to its high morbidity and death rates and association with antibiotic treatments, C. difficile is a multi-resistant pathogen that is known as the most common cause of diarrhea in healthcare settings. It is also one of the most significant public health problems. While according to the National Center for Disease Control and Prevention, C. difficile is estimated to be responsible for over 500,000 infections and 29,000 deaths in the U.S. Furthermore, according to the same source it was estimated that about 152,905 clostridioides difficile infection cases were found in Europe in the year 2022 and 8,382 clostridioides difficile infection-related deaths each year in Europe. Even though clostridioides difficile infection can affect people of all ages, the elderly are known to be particularly vulnerable to this infection. Hence, due to rising prevalence of the clostridioides difficile infection, demand for drugs for the treatment of clostridioides difficile infection increased eventually.
Global Clostridioides Difficile Infection Treatment Market Segmentation:
The global clostridioides difficile infection treatment market report is segmented into drug type, route of administration, and distribution channel
Based on Drug Type, the market is segmented into Vancomycin, Metronidazole, Fidaxomicin, and Others (late-phase drugs, etc.). Out of which, Fidaxomicin is expected to dominate the clostridioides difficile infection treatment market during the forecast period and this is attributed to market players focused on getting approval for the fidaxomicin drug to launch the new drug product in the market. For instance, in January 2020, Merck & Co., Inc., biopharmaceutical companies, based in the U.S. received the U.S. Food and Drug Administration (FDA) approval for the DIFICID (fidaxomicin) indicated for the treatment of the Clostridioides (formerly Clostridium) difficile-associated diarrhea (CDAD) in children aged six months and older.
Based on Route of Administration, the market is segmented into oral and injectable. Out of which, the oral segment is expected to dominate the market over the forecast period and this is attributed due to increasing approval by the regulatory bodies for the oral doses used in treating clostridioides difficile infection. For instance, in March 2020, the U.S. Food and Drug Administration (FDA) approved the New Drug Application (sNDA) for DIFICID tablets and oral suspension for the treatment of Clostridioides (formerly Clostridium) difficile-associated diarrhea.
Based on Distribution Channel, the market is segmented into hospital pharmacies, retail pharmacies, and online pharmacies. Out of which, hospital pharmacies segment is expected to dominate the market over the forecast period and this is attributed due to the increasing prevalence of clostridioides difficile infection. For instance, in March 2022, according to the data published by the Centers for Disease Control and Prevention, it was estimated that around 15% of the U.S. population is infected by the clostridioides difficile infection every year and around 223,900 cases of the clostridioides difficile infection were found to be hospitalized in the year 2021.
Among drug type segment, late phase drugs has the highest potential due to high demand and increased research and development activities for launching drugs used in the treatment of clostridioides difficile infection. For instance, in February 2020, Ferring Pharmaceuticals Company, a pharmaceutical company, announced the completion of enrollment of the pivotal Phase 3 clinical trial for RBX2660, an investigational treatment aimed at breaking the cycle of reoccurrence Clostridioides difficile (C. diff) infection.
Global Clostridioides Difficile Infection Treatment Market Cross Sectional Analysis:
In drug type segment, Fidaxomicin is expected to be a dominant segment in North America due to the increasing approval of the Fidaxomicin drug in the North America. For instance, Merck & Co., a pharmaceutical company, received the U.S. Food and Drug Administration (FDA) approval for the Dificid (fidaxomicin) tablets for the treatment of Clostridioides (formerly Clostridium) difficile-associated diarrhea (CDAD) in children aged six months and older.
In distribution channel segment, hospital pharmacies segment is dominant in Europe due to increasing prevalence of the clostridioides difficile infection in the European region. For instance, in September 2020, according to the data published by the National Center for Biotechnology Information, it was estimated that 56% population of the U.K. was suffering from clostridioides difficile infection in the year 2020. Furthermore, in October 2022, according to the data published by the European Centre for Disease Prevention and Control, the prevalence of the clostridioides difficile infection from the last 12 months (March 2021- March 2022) in England was estimated to be 26.8 per 100,000 population.
Global Clostridioides Difficile Infection Treatment Market: Key Developments
In October 2022, Acurx Pharmaceuticals, Inc., a biotechnology company, presented the poster as well as an oral presentation at the Infectious Disease Society of America (IDSA) IDWeek 2022 Conference highlighting new information about its lead antibiotic ibezapolstat relating to its selectivity against gram-positive gut microbiota including clostridioides difficile infection, C. coccoidies infection, and C. leptum infection groups treatment.
In November, 2022, the Combatting Bacterial Resistance in Europe Clostridioides Difficile Infection (COMBACTE-Clostridioides Difficile Infection), a European clinical research organization, celebrated November month as Clostridioides difficile awareness month.
In October, 2022, McGill University Health Centre/Research Institute of the McGill University Health Centre, in collaboration with the Canadian Institutes of Health Research (CIHR), a federal agency, initiated the clinical trial study “Initial Vancomycin Taper for the Prevention of Recurrent Clostridium Difficile Infection (TAPER-V)”. The Canadian Institutes of Health Research (CIHR) announced the positive phase 2 trial in October 2022, and the study is currently in the Phase 3 trial, which is estimated to get completed in March 2024.
Global Clostridioides Difficile Infection Treatment Market: Restraint
Huge investments required for drug development
Since there are currently just a few antibiotics that can treat Clostridioides Difficile Infection s, many pharmaceutical companies are now concentrating on creating non-antibiotic alternatives. The creation of a new medicine class will present several prospects for market expansion, but it will also unavoidably necessitate substantial investments in the research and development of novel therapies. The extremely high investment needs can serve as a restraint to market expansion. However, the collaboration, partnership, and acquisition between the key players, major manufacturers, and several funding organizations can help in the investment required in the research and development activities for drug development.
Low awareness about clostridioides difficile infection symptoms and associated effects
The initial symptoms of clostridioides difficile infection are normal as stomach cramps, a high temperature (fever), and loss motions, which is difficult to identify the clostridioides difficile infection initial stage. Viruses, bacteria, and protozoa are all possible differential diagnoses for infectious diarrhea. The symptoms of a viral infection include nausea, vomiting, occasional headaches, fever, watery diarrhea, and widespread or periumbilical stomach cramps. Viral infections are typically self-limiting. Weight loss, loose stools, meteorism, hyperperistalsis, perianal itching, wheezing, and rectal prolapse are typical signs of protozoal agents, whereas fever, blood and/or mucus in the stool, small-volume stools, and suprapubic pain are indicators of bacterial agents. Hence, the launch of new diagnostic testing methods for clostridioides difficile infection and increasing awareness programs for symptoms related to clostridioides difficile infection can help in getting the correct direction for treatment options at the right time.
Global Clostridioides Difficile Infection Treatment Market: Key Players
Major players operating in the global clostridioides difficile infection treatment market include Novartis AG, Baxter, ANI Pharmaceuticals, Inc., Mylan N.V., Akorn, Sun Pharmaceutical Industries Ltd., Merck & Co., Inc., B. Braun Medical Inc., Teva Pharmaceutical Industries Ltd., Hikma Pharmaceutical PLC, Perrigo Pharmaceutical., Apotex Inc., AbbVie Inc., Ferring Pharmaceuticals, Fresenius Kabi USA., Pfizer Inc., Strides Pharma Science Limited., Sanofi., AstraZeneca., Eli Lilly and Company, Actelion Pharmaceuticals Ltd., and Astellas Pharma.
Definition: Clostridioides difficile Infection is known as the infection in the colitis resulted from disruption of normal, healthy bacteria in the colon. Clostridioides difficile Infection can also be transmitted from person to person by spores.
Share
Share
About Author
Ghanshyam Shrivastava - With over 20 years of experience in the management consulting and research, Ghanshyam Shrivastava serves as a Principal Consultant, bringing extensive expertise in biologics and biosimilars. His primary expertise lies in areas such as market entry and expansion strategy, competitive intelligence, and strategic transformation across diversified portfolio of various drugs used for different therapeutic category and APIs. He excels at identifying key challenges faced by clients and providing robust solutions to enhance their strategic decision-making capabilities. His comprehensive understanding of the market ensures valuable contributions to research reports and business decisions.
Ghanshyam is a sought-after speaker at industry conferences and contributes to various publications on pharma industry.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients